- Open access
- Published: 03 June 2023

Human and environmental impacts of nanoparticles: a scoping review of the current literature
- Elizabeth Adjoa Kumah 1 ,
- Raoul Djou Fopa 2 ,
- Saeed Harati 2 ,
- Paul Boadu 3 ,
- Fatemeh Vida Zohoori 4 &
- Tannaz Pak 2
BMC Public Health volume 23 , Article number: 1059 ( 2023 ) Cite this article
17k Accesses
41 Citations
6 Altmetric
Metrics details
Use of nanoparticles have established benefits in a wide range of applications, however, the effects of exposure to nanoparticles on health and the environmental risks associated with the production and use of nanoparticles are less well-established. The present study addresses this gap in knowledge by examining, through a scoping review of the current literature, the effects of nanoparticles on human health and the environment. We searched relevant databases including Medline, Web of Science, ScienceDirect, Scopus, CINAHL, Embase, and SAGE journals, as well as Google, Google Scholar, and grey literature from June 2021 to July 2021. After removing duplicate articles, the title and abstracts of 1495 articles were first screened followed by the full-texts of 249 studies, and this resulted in the inclusion of 117 studies in the presented review.
In this contribution we conclude that while nanoparticles offer distinct benefits in a range of applications, they pose significant threats to humans and the environment. Using several biological models and biomarkers, the included studies revealed the toxic effects of nanoparticles (mainly zinc oxide, silicon dioxide, titanium dioxide, silver, and carbon nanotubes) to include cell death, production of oxidative stress, DNA damage, apoptosis, and induction of inflammatory responses. Most of the included studies (65.81%) investigated inorganic-based nanoparticles. In terms of biomarkers, most studies (76.9%) used immortalised cell lines, whiles 18.8% used primary cells as the biomarker for assessing human health effect of nanoparticles. Biomarkers that were used for assessing environmental impact of nanoparticles included soil samples and soybean seeds, zebrafish larvae, fish, and Daphnia magna neonates.
From the studies included in this work the United States recorded the highest number of publications ( n = 30, 25.64%), followed by China, India, and Saudi Arabia recording the same number of publications ( n = 8 each), with 95.75% of the studies published from the year 2009. The majority of the included studies (93.16%) assessed impact of nanoparticles on human health, and 95.7% used experimental study design. This shows a clear gap exists in examining the impact of nanoparticles on the environment.
• While nanoparticles are beneficial in a range of applications, they pose significant threats to humans and the environment.
• Immortalised cell lines are mostly used as biomarkers to assess human health effect of nanoparticles.
• Biomarkers such as soil samples and zebrafish larvae are used to investigate the environmental effect of nanoparticles.
• This work has revealed the toxic effects of nanoparticles to include production of oxidative stress, DNA damage, apoptosis, cell death, and induction of inflammatory responses.
Peer Review reports
Introduction
Importance and meaning.
Nanoparticles are small particles ranging from 1 to 100 nm (nm) in size [ 1 ]. They are used in a wide range of applications and can be grouped into four types: 1) inorganic-based nanoparticles, 2) carbon-based nanoparticles, 3) organic/polymer nanoparticles, and 4) composite-based nanoparticles [ 2 ]. Inorganic-based nanoparticles are made up of different metal and metal oxides. Examples of metal-based inorganic nanoparticles include aluminium, silver, gold, zinc, lead, iron, cadmium, and copper, whereas examples of metal oxide-based inorganic nanoparticles include aluminium oxide, copper oxide, iron oxide, silica, zinc oxide, titanium oxide, and magnesium aluminium oxide. Carbon-based nanoparticles include fullerene, graphene, multi- and single-walled carbon nanotubes, carbon black, and carbon fibres. Organic-based nanoparticles are derived from organic materials without carbon, for example, liposome, dendrimers, cyclodextrin, and micelle, whereas composite nanoparticles are made from combinations of metal oxide-based, metal-based, organic-based, and/or carbon-based nanoparticles.
In recent years, nanoparticles have gained increasing attention due to their use in consumer products, medicine, soil, and aquatic environments. For example, nanoparticles have been used for textiles [ 3 ], water treatment [ 4 ], environmental remediation [ 5 , 6 , 7 ], cancer therapy [ 8 ], radiology [ 9 ], and cosmetics [ 10 ]. This growing attention and extensive usage of nanoparticles is due to specific novel characteristics exhibited by such particles, which results from their small size and large surface area [ 11 ]. These unique qualities, while advantageous, pose certain risks to living organisms.
The harmful effects of nanoparticles
The small sizes of nanoparticles give them the ability to permeate physiological barriers of living organisms, causing harmful biological reactions. Nanoparticles are known to enter the human body through the lung, intestinal tract, or skin, and can be toxic to the brain, cause lung inflammation and cardiac problems [ 12 ]. In fact, certain nanoparticles have been found to cause permanent cell damage through organ injury and oxidative stress, due to their size and composition. In a study by Magrez et al. [ 13 ] to assess the toxic effect of carbon-based nanoparticles on lung cancer cells, the authors reported findings suggesting that carbon-based nanoparticles cause size-dependent cytotoxicity. The level of toxicity of nanoparticles is suggested to be dependent on factors such as composition of the nanoparticle, size, surface functionality, crystallinity, and aggregation [ 14 ]. Moreover, the toxicity of a nanoparticle in an individual is dependent on the genetic make-up of that individual, which is determined by the individual's ability to adapt and respond to toxic substances.
The gaps of previous studies
There are growing concerns regarding the toxicologic effects of nanoparticles, and frequent exposure to nanoparticles is regarded as a public health threat [ 15 ]. While there is extensive evidence about the benefits of nanoparticles, as well as the potential health and environmental risks associated with its production and use, current understanding of the impact of nanoparticles exposure to human health and the environment is limited. The current review seeks to explore, through a scoping review of the current literature, the effects of nanoparticles on human health and the environment. This review is unique as it adopts a systematic scoping approach to explore the current literature on the health risks posed by the manufacture, distribution, and use of nanoparticles. Published studies in this area have mainly used a narrative literature review approach [ 2 , 16 , 17 ].
Objective and research questions
The objective of this review is to map the distribution of the current literature on the human and environmental impacts of nanotoxicity. Specifically, this scoping review will be guided by the following research questions:
What is the relative distribution of the current literature on the human and environmental impact of nanotoxicity?
Which exposure pathways and nanoparticles have been researched and which have not?
What biomarkers have been used in assessing the human and environmental impact of exposure to nanoparticles?
This scoping review was conducted and reported in accordance with the Joanna Briggs Institute Reviewers Manual [ 18 ]. The following steps were followed:
Defining and aligning the objectives and research questions
Developing and aligning the inclusion criteria with the objectives and research questions
Describing the planned approach to evidence searching and selection
Searching for the evidence
Extracting the evidence
Charting the evidence
Summarising the evidence in relation to the objectives and research questions
The Preferred Reporting of Items for Systematic Reviews and Meta-Analysis (PRISMA) statement was used to summarise the screening process. The protocol of this review has been registered with the Open Science Framework [ 19 ].
Search strategy
The aim of the search strategy was to find both published and unpublished studies that have examined the effect of nanotoxicity on human health and the environment. Search terms consisted of a combination of key terms and concepts in the objective and research questions, using the Boolean operators, 'AND', and 'OR' as follows:
(nanomaterials OR nanoparticles OR nanostructures) AND (toxicity OR health) AND (“biomarker* of exposure” OR biomarker OR exposure) AND (human OR environment).
The search was limited to peer-reviewed articles published from the year 2000. This was to enable us to study the current literature (research conducted over the last 2 decades). The search was limited to primary studies published in the English language due to difficulties with language translation.
Table 1 below presents a list of the databases, grey literature, and search engines that were searched for eligible papers. The reference list of all included papers was also searched for additional papers on the subject matter.
For the database searches, a master search strategy was first developed using the Medline database, this was then modified for the other databases. The supplementary material file presents the Medline search history. The literature search was conducted between 1 st June 2021 and 31 st July 2021.
Reference management
All search results were imported into an Endnote library to help manage references and to remove duplicate articles. Once duplicates were removed, the search results were exported from Endnote into Covidence (a web-based software platform that streamlines the production of scoping/systematic reviews) for screening. The Covidence software was also useful in identifying and deduplicating articles that could not be identified by Endnote.
Selection criteria
The following criteria were used to identify eligible articles for inclusion in the review.
Inclusion criteria
Types of participants.
Studies that have assessed the human and environmental impacts of nanotoxicity were considered for inclusion in this review. Human participants included children and/or adults of any age, gender, or ethnicity. Studies involving the use of animals as biomarkers for assessing the environmental impact of nanotoxicity were also considered for inclusion.
Studies that have examined the impacts of nanotoxicity as well as the biomarkers for assessing exposure to nanoparticles were eligible for inclusion in this review. While all types of nanoparticles were considered for inclusion, attention was given to studies involving metallic (oxides, pure metal) and carbonaceous (fullerenes, carbon nanotubes, and graphene) nanoparticles. This is mainly due to these particles being widely produced and used [ 20 ], therefore, they are considered the most relevant for public health.
Studies from any geographical location aimed at assessing the human and/or environmental impact of nanotoxicity were considered eligible for inclusion. Studies whose full texts were in a language other than English were excluded because there were no available translators.
Study types
We included all original primary research (both quantitative and qualitative), including, but not limited to randomised controlled studies, quasi-experimental studies, surveys, retrospective and prospective cohort studies, case studies, and phenomenological studies.
Exclusion criteria
The following exclusion criteria were applied to the title and abstract, as well as the full-text review stage:
Irrelevant problem/focus: studies that have not examined the human and/or environmental impact of nanotoxicity, or the biomarkers for assessing exposure to nanoparticles
Irrelevant type of study: review reports or studies that did not contain any original research
Selection of studies
We employed a two-step screening process to assess search results for eligible studies. The first level involved screening of the titles and abstracts and was done independently by two reviewers (EK and RF). The next step was carried out independently by three reviewers (EK, RF, and SH) and involved screening of the full-texts of potentially eligible papers. Disagreements between reviewers were resolved through discussions and consensus. Where disagreements persisted, a third reviewer (TP or FVZ) was consulted.
Data charting
We developed a standardised data extraction form in the Covidence software for data extraction. The form was designed to collect the following information from included studies: year of publication, aim/objective of study, study design, country, type of nanoparticle, application of the nanoparticle, major exposure route(s), biomarker/model used, how biomarker was obtained, and study outcome(s).
The developed data extraction form was pilot-tested using 10% of the included articles before beginning the actual data extraction. Data extraction was done by one reviewer (PB, RF, or SH) and verified by another (EK, TP, or FVZ), using the Covidence software.
Data synthesis
The extracted data was first exported into Excel for editing and to check for accuracy. The edited data was then exported from Excel into SPSS (version 26) to aid with data synthesis. Descriptive statistics was used to report included studies by their characteristics and outcome measures, described below.
Characteristics of included studies
Year of publication: studies were grouped based on their year of publication . As stated earlier, this included studies published from the year 2000 to July 2021 (the date of completion of literature searches).
Country in which study was conducted : to assess the distribution of the current literature on human and environmental impact of nanotoxicity, the countries in which eligible studies were conducted were classified into six regions based on the World Bank’s classification of countries. This included: East Asia and Pacific, Europe and Central Asia, Latin America and Caribbean, Middle East and North Africa, North America, South Asia, and Sub-Saharan Africa (World Bank Group, 2018).
Study design : randomised controlled trial, non-randomised controlled trial, cohort study, experimental study, case control study, longitudinal study, uncontrolled before and after studies.
Impact/effect assessed : human health and/or environment
Outcome measures
Type of nanoparticle : This was divided into four groups: 1) inorganic-based nanoparticles, 2) carbon-based nanoparticles, 3) organic nanoparticles, and 4) composite-based nanoparticles two groups, metallic (oxides, pure metal) and carbonaceous (fullerenes and carbon nanotubes) particles.
Biomarker or model used in assessing human and/or environmental exposure : primary cell or immortalised cell line
Effect/impact on human health and/or the environment
A narrative synthesis was then used to further explore findings.
Search results
The database searches resulted in 1553 papers (presented in Fig. 1 ): Medline ( n = 1,381); ScienceDirect ( n = 0); Sage Journals Online ( n = 50); Campbell Collaboration ( n = 0); Cochrane Collaboration ( n = 0); Embase ( n = 5); Scopus ( n = 6); Web of Science ( n = 50); CINAHL ( n = 61). Google and Google Scholar searches yielded 100 results, and no article was obtained from grey literature searches. Following removal of duplicate articles, the titles and abstracts of 1495 articles were screened to assess their eligibility for inclusion, which resulted in the exclusion of a total of 1246 articles as they did not meet the inclusion criteria. As such, the full texts of 249 articles were assessed for eligibility. Following this stage, a total of 132 articles were excluded for several reasons (see Fig. 1 ), whereas 117 studies qualified for inclusion in the review.
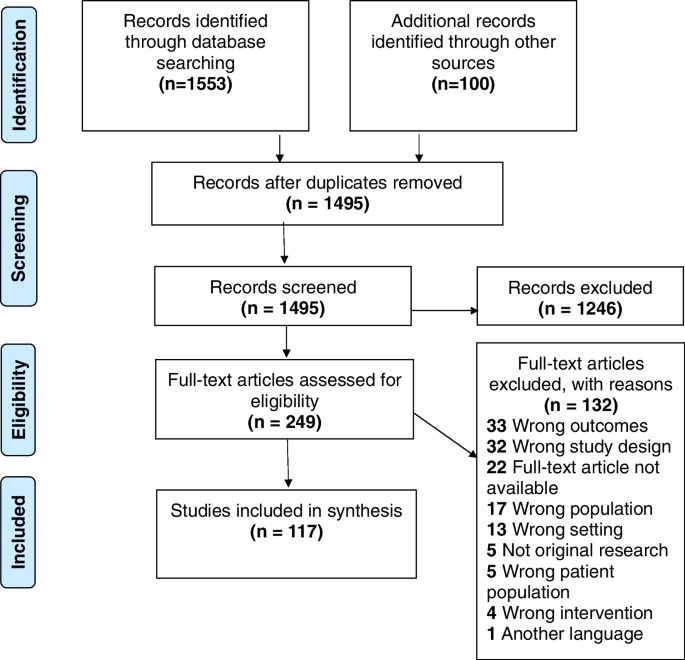
Study flow diagram (adapted from Moher et al., 2009)
The studies included in this review originated from 23 countries across several continents, with the majority of the studies originating from Europe and Central Asia ( n = 50). Nevertheless, the United Sates recorded the highest number of publications ( n = 30), followed by China, India, and Saudi Arabia recording the same number of publications ( n = 8). The lowest number of studies ( n = 1 each) originated from Argentina, Czech Republic, Egypt, Mexico, Pakistan, Poland, and Russia. There were no studies recorded from Sub-Saharan Africa. Figure 2 presents a classification of the included studies by region.
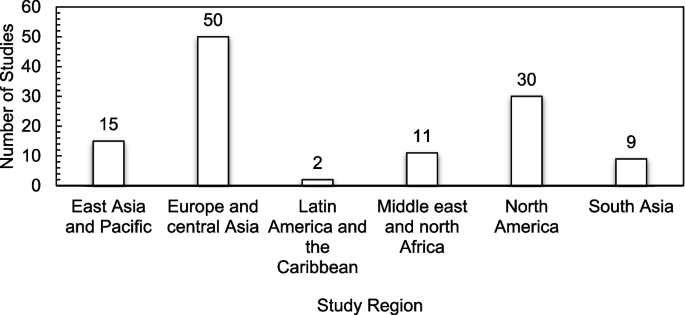
Classification of Studies by Region
Included studies were published between the year 2006 and 2021, with a high proportion of the articles (95.75%) published from the year 2009. However, the year 2020 recorded the highest number of publications ( n = 15; 12.82%), followed by 2016 ( n = 14; 11.97%). Table 2 below presents the number of publications per year.
The majority of the studies used an experimental study design ( n = 112, 95.7%), with only 5 (4.3%) studies employing a cross-sectional design. Regarding the type of impact/effect of nanoparticle assessed, a vast majority of the studies assessed impact on human health ( n = 109), 5 of the studies assessed effects on the environment, with only 3 studies assessing both human and environmental health impact (Fig. 3 ).
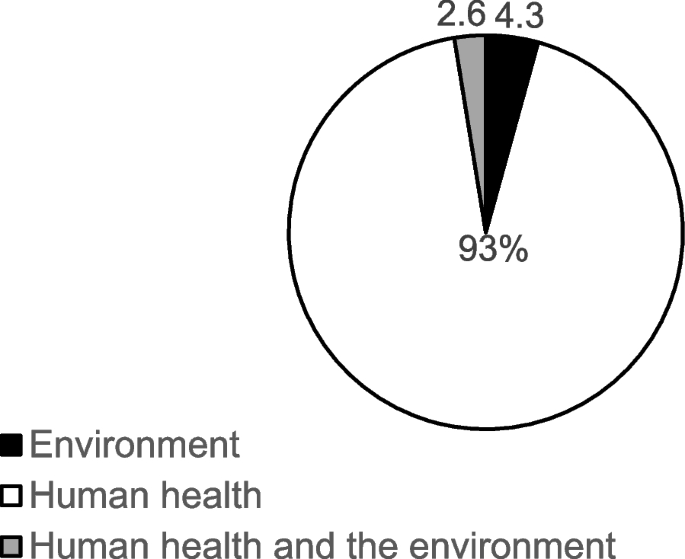
Effect/impact of nanoparticles on human/environmental health
Just over 65% ( n = 77) of the included studies investigated the human and/or environmental effect of inorganic-based nanoparticles. The inorganic-based nanoparticles that were investigated include, but not limited to, bismuth oxide (Bi 2 O 3 ), silicon dioxide (SiO 2 ), copper oxide (CuO), zinc oxide (ZnO), titanium dioxide (TiO 2 ), silver (Ag), gold (Au), platinum (Pt), iron oxide (Fe 2 O 3 ), cerium oxide (CeO 2 ), cobalt oxide (Co 3 O 4 ), aluminium oxide (Al 2 O 3 ), molybdenum trioxide (MoO3), magnesium oxide (MgO), nickel oxide (NiO), chromium oxide (Cr 2 O 3 ), tungsten oxide (WO 3 ), yttrium oxide (Y 2 O 3 ), and manganese oxide (Mn 2 O 3 ).
Thirty-five (29.9%) studies reported on carbon-based nanoparticles (including single and multi-walled carbon nanotubes (SWCNTs/MWCNTs), graphene oxide (GO), and graphene nanoplatelets, GNP). Three studies [ 21 , 22 , 23 ] reported on both inorganic- and carbon-based nanoparticles; one study [ 24 ] reported on both inorganic-based and polymer nanoparticles (i.e., Titanium dioxide, terbium-doped gadolinium, and polylactic-co-glycolic acid, PLGA), whereas another study [ 25 ] investigated the effect of Poly lactic-co-glycolic acid (a polymer) nanoparticle on the environment.
The most investigated nanoparticles were ZnO ( n = 25), followed by MWCNTs ( n = 20), TiO 2 ( n = 16), CeO ( n = 15), SWCNTs and Fe 2 O 3 ( n = 14), and SiO 2 ( n = 12). The least studied nanoparticles include Pt, Au, MgO, MoO 3 , WO 3 , Carbon Black (CB), and GNP with only one report available.
A significant number ( n = 90, 76.9%) of the included studies used immortalised cell lines as the biomarker for assessing the human health effect of nanoparticles. Examples of the immortalised cell lines that were used include the human hepatocarcinoma cell line (HepG2), human (alveolar) epithelial A549 cell line with human monocyte-derived dendritic cells (MDDCs) and macrophages (MDMs), Melanoma cells and human foreskin fibroblasts, human airway epithelial (BEAS-2B) cells, human bronchial epithelium (BEAS-2B) cells, human neuroblastoma SHSY5Y cell line, human keratinocyte (HaCaT) cell line, and MCF-7 cell line, which is a human breast cancer cell line with oestrogen, progesterone and glucocorticoid receptors. Immortalised cell lines were mostly purchased/obtained from organisations such as the American Type Culture Collection (ATCC, Manassas, VA, USA).
Twenty-two studies used primary cells obtained from study participants/volunteers. Examples of the primary cells that were used as biomarkers by included studies are human bone marrow mesenchymal stem cells (hBMMSCs) taken from the iliac crest of human donors, human lymphocytes (blood), and human dermal fibroblasts which were isolated by the outgrowth method using infant foreskins obtained after circumcision. Workplace air samples have also been used to investigate workplace exposures to graphene nanoplatelets [ 26 ]. Five studies [ 25 , 27 , 28 , 29 , 30 ] that reported on the environmental effect of nanoparticles used a variety of biomarkers, including soil samples and soybean seeds, Allium cepa bulbs, zebrafish larvae, seedlings of buckwheat, Nitrosomonas europaea KCTC 12270 bacterium (an ammonia-oxidizing bacterium) and Nitrospira moscoviensis (a nitrite-oxidizing bacterium), as well as aquatic species including Daphnia magna neonates, fish, and Carp (Cyprius carpio). The studies included in this review reported several toxicities associated with the production and application of nanoparticles. The most reported health impact of nanoparticles was found to be decreased cell viability and/or cell death (observed by twenty-nine studies). Twenty-eight studies also noted reactive oxygen species generation as a result of exposure to nanoparticles, especially to CNT ( n = 7), ZnO ( n = 7), SiO 2 ( n = 5), and TiO 2 ( n = 4). The third commonly observed health impact was dose-dependent oxidative stress in the biomarkers ( n = 25), particularly, in cases of exposure to SiO 2 ( n = 5), ZnO ( n = 5), Fe 3 O 4 ( n = 4), CeO 2 ( n = 3), and CuO ( n = 3). In addition, there were sixteen reports regarding DNA damage after exposure to nanoparticles, mainly for ZnO ( n = 4) and MWCNTs ( n = 3). Table 3 presents a comprehensive outline of the effects (human health and the environment) reported by each of the included studies. These are further explored in the ensuing section.
The objective of this scoping review was to ascertain the distribution of the current literature on the human and environmental impacts of nanoparticles. Specifically, in this review, we synthesised evidence regarding the exposure pathways and types of nanoparticles that have been researched and the ones that have not, as well as the biomarkers that have been used in assessing human and environmental impact of exposure to nanoparticles.
While the majority of studies originated from Europe and Central Asia, the United States of America (USA) alone recorded the highest number of publications. This finding is not surprising, as the USA has continuously fostered the development of nanotechnology through significant investments in research and development in this area. In 2016, the USA was projected to account for almost one-third of total global nanotechnology research funding [ 136 ]. Moreover, the USA and the European Union have over the years taken a committed approach towards enhancing the health and safety of nanoparticles [ 137 ]. As part of this commitment, annual meetings are held, where researchers discuss topics relating to nano-safety, as well as funding priorities and research needs.
While there have been some investments in nanotechnology research in African countries (including Egypt and South Africa), a recent publication by the United Nations Economic Commission for Africa (UNECA) indicates that the African continent, relative to other continents, is lagging behind with regards to nanotechnology research [ 138 ]. This assertion is consistent with the findings of this review, which found only one study originating from North Africa (Egypt), with no study conducted in Sub-Saharan Africa.
Over the past two decades, there have been increasing public awareness of nanotechnology and a growing concern about its commercial applications [ 139 ]. This has led to rapidly increasing scientific publications in this field, especially from early 2000s [ 140 ]. It is, therefore, not surprising that the studies included in this scoping review were published from the year 2006. Indeed, a literature search of nanotechnology publications by Huang et al. [ 140 ] revealed over 50,000 publications for the year 2006.
Although the included studies investigated a wide range of nanoparticles, most of them focused on inorganic-based nanoparticles (e.g., zinc oxide, titanium dioxide, copper oxide, and silica), followed by carbon-based nanoparticles (e.g., carbon-nanotubes, fullerenes, and graphene) (Table 3 ). This finding is consistent with previous reviews that have reported extensive investigation into the impact of inorganic-based and/or carbon-based nanoparticles [ 141 , 142 ]. These nanoparticles may have gained attention due to their extensive production and usage. In addition to their use for cancer treatment, inorganic and carbon-based nanoparticles provide significant benefits in photothermal therapy, diagnosis, tissue engineering, imaging contrast agents, and sensing applications [ 143 ]. This is due to their unique physical and chemical properties (such as electrical, thermal, structural, mechanical, and optical diversity), which make them stronger, flexible, and more electrically conductible towards several biological entities [ 141 , 144 ]. The advantages of, for example inorganic-based nanoparticles, including their high reactivity, small size and good capacity have been found to induce adverse harmful effects in both humans and the environment.
In this review, a number of approaches were used by included studies to assess the toxicity of nanoparticles. However, the majority of the studies applied the in vitro method, perhaps because in vitro studies are time saving and cost-effective. Nonetheless, the in vitro approach has been criticised by researchers (e.g., Bahadar et al. [ 145 ]) for producing varying results in different laboratories.
The included studies used differing methods in assessing cytotoxicity and genotoxicity: cell membrane integrity was assessed with Lactate dehydrogenase (LDH) assays [ 44 , 57 , 116 ]; cell viability was assessed using tetrazolium reduction assays [ 82 , 83 , 90 , 116 ]; apoptosis was assessed using immunohistochemistry biomarkers [ 60 , 65 , 86 ]; electron microscopy was used to assess intracellular localisation of nanoparticles [ 34 , 106 ]; and cell inflammation was estimated using chemokines biomarkers (i.e., IL-8, TNF- α, and IL-6) [ 146 ]. Compounds such as MTT, XTT, MTS, and WST-1 are used to detect viable cells [ 147 ]. However, in the current review, most of the studies employed MTT tetrazolium assays for investigating cell toxicity [ 47 , 49 , 50 , 58 , 116 ]. Similar findings have been reported by Bahadar et al. [ 145 ] who conducted a review on the toxicity of nanoparticles.
The human impact of nanoparticles
Most of the studies in this review focused on assessing the characteristics of nanoparticles, as well as the impact of nanoparticles on, particularly, human health. In recent years, there have been promising results from the application of nanoparticles to human health, especially in cancer treatment. This is due to the potential of nanoparticles to provide innovative solutions to curb the limitations of traditional treatment methods, including radiotherapy and chemotherapy [ 148 ]. Relative to conventional cancer treatment methods, nanoparticle-based drug delivery systems have been shown to have significant advantages in a) drug resistance, b) correctly targeting tumour cells, c) having good pharmacokinetics, and d) reduction of treatment side effects [ 149 ]. Notwithstanding these benefits, however, nanoparticles have potential harmful effects, and there are controversies about their safe use in humans [ 139 ]. This has undoubtedly led to the rapidly growing number of studies investigating the human health impact of nanoparticles, as was revealed in this review.
The majority of the studies (n = 90) in this review used immortalised cell lines as the biomarker for assessing human health impact of nanoparticles, and only 22 studies used primary cells as biomarkers. Immortalised cell lines have mostly been used for nano-safety studies because, relative to primary cells, they are generally less expensive, readily accessible, and easier to cultivate [ 150 ]. However, the type of cell that is used as biomarker for nano-safety studies is of great importance since this may have an impact on the general outcome of studies [ 151 ]. Cancer cell lines, for example, have a disturbed anti-apoptotic balance, and have undergone transformation in metabolism, which impacts their ability to sustain their high rate of proliferation [ 152 ]. As such, using these cells may have an impact on study findings. Nonetheless, the use of primary cells in nano-safety studies, are not without limitations. Primary cells have limited lifespan in vitro and can suffer from clonal changes.
In using immortalised cell lines, several studies [ 153 , 154 ]) have reported variations in findings regarding nanoparticle-induced effects in cell lines obtained from different species or tissues. For example, Zhang et al. [ 153 ] and Mukherjee et al. [ 154 ] investigated the effect of exposure to silver nanoparticle on mammalian cells. Zhang et al. [ 153 ] used epithelial cells and microphages, and Mukherjee et al. [ 154 ] used the human dermal and cervical cell lines as biomarkers. Mukherjee et al. [ 154 ] reported nanoparticle-induced cytotoxicity such as elevated levels of oxidative stress, cell membrane damage, and glutathione depletion, whereas Zhang et al. [ 153 ] reported effects including changes in antioxidant defence and metallothionein. Moreover, while Ekstrand-Hammarstrom et al. [ 155 ] and Kermanizadeh et al. [ 156 ] have compared the effect of nanoparticles on immortalised cell lines versus primary cells of the same species and tissues, available data regarding the relative effectiveness of these two types of cells are unclear. Therefore, it is difficult to make explicit conclusions as to which of these two types of cells can be used as a reliable biomarker for nano-safety studies.
This review has revealed that humans are exposed to nanoparticles through inhalation, ingestion, or dermal route. After their exposure, nanoparticles induce toxic effects such as production of oxidative stress at the exposure site, inflammation, DNA damage, and cell death [ 87 , 88 ]. For instance, exposure of human neuroblastoma (Sh-sy5y) cells to inorganic nanoparticles, such as titanium dioxide, silica dioxide, and silver are associated with induction of neurotoxicity, membrane damage, reaction oxygen specie formation, decrease in cell viability, and autophagy dysfunction [ 40 ]. Similarly, exposure to carbon-based nanoparticles such as single and multi-walled carbon nanotubes reduce cell viability, as well as induce changes in cell structure, cell cycle, and cell-to-cell interactions in human lung epithelial cells (BEAS-2B) [ 107 ].
The environmental impact of nanoparticles
The findings of this scoping review indicate a gap in the literature regarding environmental impact of nanoparticles. Out of the 117 included studies, only 5 had assessed the environmental impact of exposure to nanoparticles. This significant gap in the scientific literature has been highlighted by authors such as Bundschuh et al. [ 157 ]. The growing production and usage of nanoparticles has undoubtedly led to a diversification of emission sources into both the aquatic and soil environment. Nanoparticles enter the environment mainly through three emission scenarios: a) released during production of nano-enabled products and raw materials, b) during application, and c) following disposal of products containing nanoparticles [ 158 ]. These emissions occur either indirectly through systems such as landfills or wastewater treatment plants, or directly to the environment. Nonetheless, nanoparticles are mostly released during the application phase and following disposal [ 159 ]. Indeed, during production, only about 2% of the production volume is emitted [ 160 ]. The studies in this review used biomarkers such as soil samples and soybean seeds, zebrafish larvae, fish, and Daphnia magna neonates. This finding is in line with a previous review by Bundschuh et al. [ 157 ], which explored the effects of nanoparticles on the environment.
Limitations of the review
In this review, every effort was made to reduce bias. The search strategy was developed by experts of the review team with many years of experience in conducting systematic/scoping reviews. A comprehensive search of multiple relevant databases and other resources was conducted by one review author (EAK) and a rerun of the searches was done after 4 weeks of the initial search. Two authors (EAK and RF or PB and SH) independently screened the search results, and disagreements between reviewers were resolved by FVZ or TP.
The main limitation of this review is that the searches were limited to studies published in the English language. This may have led to the exclusion of potentially relevant papers published in other languages. Also, searches were restricted to studies published from the year 2000, which may have led to the omission of potentially relevant papers.
Conclusions
This review has provided an extensive synthesis of the current literature on the effects of nanoparticles on human health and the environment. The review has shown that while nanoparticles are beneficial in a range of applications, they pose significant threats to humans and the environment. Through the use of several biological models and biomarkers (e.g., human bronchial epithelial cells (Beas-2), soil samples, and soybean seeds), the included studies revealed the toxic effects of nanoparticles, with the most investigated nanoparticles being Zinc Oxide, MWCNTs, Titanium Dioxide, Cerium Oxide, SWCNTs, Ferric Oxide, and Silicon Dioxide. The main health impacts of nanoparticles identified in this review are decreased cell viability, cell death, reactive oxygen species generation, production of oxidative stress (dose-dependent), DNA damage, apoptosis, and induction of inflammatory responses.
This review has revealed a significant gap in the scientific literature regarding environmental impact of nanoparticles of all types. Future studies should be directed at investigating the impact of the various types of nanoparticles on the aquatic, terrestrial, and soil environment. The findings from this review have also shown limited data regarding the relative effectiveness of immortalised cell lines and primary cells as biomarkers in nano-safety studies. Future research should focus on evaluating the effectiveness of these two types of cells, in order to determine the cell that can be used as a reliable biomarker for nano-safety studies. There is also the need for future studies in this area to focus on exploring the toxic effects of Platinum, Gold, Magnesium Oxide, Molybdenum Trioxide, Tungsten trioxide, and Carbon Black nanoparticles, as findings from this review has shown that these nanoparticles are least researched. The findings of this review will be useful to policy makers and stakeholders in assessing the potential effects of nanoparticles.
Availability of data and materials
All data generated or analysed during this study are included in this published article and in the presented supplementary material file.
Jiao H, Barakat N. Balanced depth and breadth in a new interdisciplinary nanotechnology course. J Educ Technol Syst. 2012;40(1):75–87.
Article Google Scholar
Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2017;12(7):908–31.
Som C, Wick P, Krug H, Nowack B. Environmental and health effects of nanomaterials in nanotextiles and facade coatings. Environ Int. 2011;37:1131–42.
Article CAS PubMed Google Scholar
Bottero JY, Rose J, Wiesner MR. Nanotechnologies: Tools for sustainability in a new wave of water treatment processes. Integr Environ Assess Manag. 2006;2:391–5.
Pak T, Archilha NL, de Lima Luz, LF. Nanotechnology-Based Remediation of Groundwater. In: Kumar C, editor. Nanotechnology Characterization Tools for Environment, Health, and Safety. Berlin, Heidelberg: Springer; 2019. p. 145–65.
Pak T, Archilha NL, Al-Imari R. Application of nanotechnology in removal of NAPLs from contaminated aquifers: a source clean-up experimental study. Clean Technol Environ Pol. 2018;20:427–33.
Article CAS Google Scholar
Pak T, Luz LFDL, Tosco T, Costa GSR, Rosa PRR, Archilha NL. Pore-scale investigation of the use of reactive nanoparticles for in situ remediation of contaminated groundwater source. Proc Natl Acad Sci. 2020;117(24):13366–73.
Article CAS PubMed PubMed Central Google Scholar
Lammers T, Hennink WE, Storm G. Tumour-targeted nanomedicines: Principles and practice. Br J Cancer. 2008;99:392–7.
Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2006;26:3995–4021.
Botta C, Labille J, Auffan M, Borschneck D, Miche H, Cabie M, et al. TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: structures and quantities. Environ Pollut. 2011;159:1543–50.
Auffan M, Bottero JY, Chaneac C, Rose J. Inorganic manufactured nanoparticles: How their physicochemical properties influence their biological effects in aqueous environments. Nanomed Nanotechnol Biol Med. 2010;5:999–1007.
CAS Google Scholar
Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39.
Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6(6):1121–5.
Mancuso L, Cao G. Acute toxicity test of CuO nanoparticles using human mesenchymal stem cells. Toxicol Mech Methods. 2014;24(7):449–54.
Yokel RA, MacPhail RC. Engineered nanomaterials: exposures, hazards, and risk prevention. J Occup Med Toxicol. 2011;6:7.
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulation. Beilstein J Nanotechnol. 2018;9:908–31.
Sahu SC, Hayes AW. Toxicity of nanomaterials found in human environment: a literature review. Toxicol Res Appl. 2017;1:2397847317726352.
Google Scholar
Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. Joanna Briggs Institute. 2020. Available from: https://doi.org/10.46658/JBIMES-20-01
Kumah EA, Zohoori V, Pak T, Harati S, Raoul FD, Boadu P. Effects of nanotechnology: A scoping review of the current literature. OSF; 2021. Available from: osf.io/w67m9.
Boczkowski J, Hoet P. What’s new in nanotechnology? Implications for public health from a brief review of the 2008 literature. Nanotoxicology. 2010;4(1):1–14.
Phuyal S, Kasem M, Rubio L, Karlsson HL, Marcos R, Skaug V, et al. Effects on human bronchial epithelial cells following low-dose chronic exposure to nanomaterials: A 6-month transformation study. Toxicol Vitro : Int J Publishe Assoc BIBRA. 2017;44:230–40.
Shalini D, Senthilkumar S, Rajaguru P. Effect of size and shape on toxicity of zinc oxide (ZnO) nanomaterials in human peripheral blood lymphocytes. Toxicol Mech Methods. 2018;28(2):87–94.
Simon-Deckers A, Gouget B, Mayne-L’hermite M, Herlin-Boime N, Reynaud C, Carrière M. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology. 2008;253(1–3):137–46.
Setyawati MI, Khoo PKS, Eng BH, Xiong S, Zhao X, Das GK, et al. Cytotoxic and genotoxic characterization of titanium dioxide, gadolinium oxide, and poly(lactic-co-glycolic acid) nanoparticles in human fibroblasts. J Biomed Mater Res, Part A. 2013;101(3):633–40.
Nishu SD, Park S, Ji Y, Han I, Key J, Lee TK. The effect of engineered PLGA nanoparticles on nitrifying bacteria in the soil environment. J Ind Eng Chem. 2020;84:297–304.
Lee JH, Han JH, Kim JH, Kim B, Bello D, Kim JK, et al. Exposure monitoring of graphene nanoplatelets manufacturing workplaces. Inhalation Toxicol. 2016;28(6):281–91.
Gaiser BK, Fernandes TF, Jepson M, Lead JR, Tyler CR, Stone V, et al. Assessing exposure, uptake and toxicity of silver and cerium dioxide nanoparticles from contaminated environments. Environ Health. 2009;8(1):S2.
Article PubMed PubMed Central Google Scholar
Ghosh M, Bhadra S, Adegoke A, Bandyopadhyay M, Mukherjee A. MWCNT uptake in Allium cepa root cells induces cytotoxic and genotoxic responses and results in DNA hyper-methylation. Mutat Res. 2015;774:49–58.
Lee S, Kim S, Kim S, Lee I. Effects of soil-plant interactive system on response to exposure to ZnO nanoparticles. J Microbiol Biotechnol. 2012;22(9):1264–70.
Yusefi-Tanha E, Fallah S, Rostamnejadi A, Pokhrel LR. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci Total Enviro. 2020;738:140240.
Ahamed M, Akhtar MJ, Khan MAM, Alrokayan SA, Alhadlaq HA. Oxidative stress mediated cytotoxicity and apoptosis response of bismuth oxide (Bi2O3) nanoparticles in human breast cancer (MCF-7) cells. Chemosphere. 2019;216:823–31.
Eom H-J, Choi J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol In Vitro. 2009;23(7):1326–32.
Bengalli R, Colantuoni A, Perelshtein I, Gedanken A, Collini M, Mantecca P, et al. In vitro skin toxicity of CuO and ZnO nanoparticles: Application in the safety assessment of antimicrobial coated textiles. NanoImpact. 2021;21: 100282.
Ferraro SA, Domingo MG, Etcheverrito A, Olmedo DG, Tasat DR. Neurotoxicity mediated by oxidative stress caused by titanium dioxide nanoparticles in human neuroblastoma (SH-SY5Y) cells. J Trace Elem Med Biol. 2020;57: 126413.
Article PubMed Google Scholar
Gambardella C, Morgana S, Bari GD, Ramoino P, Bramini M, Diaspro A, et al. Multidisciplinary screening of toxicity induced by silica nanoparticles during sea urchin development. Chemosphere. 2015;139:486–95.
Oh JH, Son MY, Choi MS, Kim S, Choi AY, Lee HA, Kim KS, Kim J, Song CW, Yoon S. Integrative analysis of genes and miRNA alterations in human embryonic stem cells-derived neural cells after exposure to silver nanoparticles. Toxicol Appl Pharmacol. 2016;299:8-23. https://doi.org/10.1016/j.taap.2015.11.004 .
Zhao L, Yu C, Lv J, Cui Y, Wang Y, Hou C, et al. Fluoride exposure, dopamine relative gene polymorphism and intelligence: A cross-sectional study in China. Ecotoxicol Environ Saf. 2021;209: 111826.
Ying E, Hwang H-M. In vitro evaluation of the cytotoxicity of iron oxide nanoparticles with different coatings and different sizes in A3 human T lymphocytes. Sci Total Environ. 2010;408(20):4475–81.
Jing X, Park JH, Peters TM, Thorne PS. Toxicity of copper oxide nanoparticles in lung epithelial cells exposed at the air–liquid interface compared with in vivo assessment. Toxicol In Vitro. 2015;29(3):502–11.
Lojk J, Repas J, Veranič P, Bregar VB, Pavlin M. Toxicity mechanisms of selected engineered nanoparticles on human neural cells in vitro. Toxicology. 2020;432: 152364.
Tsai S-M, Duran-Robles E, Goshia T, Mesina M, Garcia C, Young J, et al. CeO2 nanoparticles attenuate airway mucus secretion induced by TiO2 nanoparticles. Sci Total Environ. 2018;631–632:262–9.
Sramkova M, Kozics K, Masanova V, Uhnakova I, Razga F, Nemethova V, et al. Kidney nanotoxicity studied in human renal proximal tubule epithelial cell line TH1. Mut Res/Gen Toxicol Environ Mutagen. 2019;845: 403017.
Bell KJ, Lansakara TI, Crawford R, Monroe TB, Tivanski AV, Salem AK, et al. Mechanical cues protect against silica nanoparticle exposure in SH-SY5Y neuroblastoma. Toxicol In Vitro. 2021;70: 105031.
Akhtar MJ, Ahamed M, Kumar S, Siddiqui H, Patil G, Ashquin M, et al. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology. 2010;276(2):95–102.
Dankers ACA, Kuper CF, Boumeester AJ, Fabriek BO, Kooter IM, Gröllers-Mulderij M, et al. A practical approach to assess inhalation toxicity of metal oxide nanoparticles in vitro. J Appl Toxicol : JAT. 2018;38(2):160–71.
Chen R, Huo L, Shi X, Bai R, Zhang Z, Zhao Y, et al. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano. 2014;8(3):2562–74.
Patil NA, Gade WN, Deobagkar DD. Epigenetic modulation upon exposure of lung fibroblasts to TiO 2 and ZnO nanoparticles: alterations in DNA methylation. Int J Nanomed. 2016;11:4509–19.
Mohamed BM, Verma NK, Prina-Mello A, Williams Y, Davies AM, Bakos G, et al. Activation of stress-related signalling pathway in human cells upon SiO2 nanoparticles exposure as an early indicator of cytotoxicity. J Nanobiotechnol. 2011;9:29.
Alshatwi AA, Subbarayan PV, Ramesh E, Al-Hazzani AA, Alsaif MA, Alwarthan AA. Aluminium oxide nanoparticles induce mitochondrial-mediated oxidative stress and alter the expression of antioxidant enzymes in human mesenchymal stem cells. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30(1):1–10.
Baber O, Jang M, Barber D, Powers K. Amorphous silica coatings on magnetic nanoparticles enhance stability and reduce toxicity to in vitro BEAS-2B cells. Inhalation Toxicol. 2011;23(9):532–43.
Corbalan JJ, Medina C, Jacoby A, Malinski T, Radomski MW. Amorphous silica nanoparticles aggregate human platelets: potential implications for vascular homeostasis. Int J Nanomed. 2012;7:631–9.
Branica G, Mladinić M, Omanović D, Želježić D. An alternative approach to studying the effects of ZnO nanoparticles in cultured human lymphocytes: combining electrochemistry and genotoxicity tests. Arh Hig Rada Toksikol. 2016;67(4):277–88.
Gurunathan S, Jeyaraj M, La H, Yoo H, Choi Y, Do JT, et al. Anisotropic platinum nanoparticle-induced cytotoxicity, apoptosis, inflammatory response, and transcriptomic and molecular pathways in human acute monocytic leukemia cells. Int J Mol Sci. 2020;21(2):440.
Hussain S, Al-Nsour F, Rice AB, Marshburn J, Ji Z, Zink JI, et al. Cerium dioxide nanoparticles do not modulate the lipopolysaccharide-induced inflammatory response in human monocytes. Int J Nanomed. 2012;7:1387–97.
Zerboni A, Bengalli R, Baeri G, Fiandra L, Catelani T, Mantecca P. Mixture effects of diesel exhaust and metal oxide nanoparticles in human lung a549 cells. Nanomaterials (Basel, Switzerland). 2019;9(9):1302.
Zielinska E, Tukaj C, Radomski MW, Inkielewicz-Stepniak I. Molecular mechanism of silver nanoparticles-induced human osteoblast cell death: protective effect of inducible nitric oxide synthase inhibitor. PLoS ONE. 2016;11(10): e0164137.
Rajiv S, Jerobin J, Saranya V, Nainawat M, Sharma A, Makwana P, et al. Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron (III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro. Hum Exp Toxicol. 2016;35(2):170–83.
Alarifi S, Ali D, Verma A, Alakhtani S, Ali BA. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int J Toxicol. 2013;32(4):296–307.
Sun J, Wang S, Zhao D, Hun FH, Weng L, Liu H. Cytotoxicity, permeability, and inflammation of metal oxide nanoparticles in human cardiac microvascular endothelial cells: cytotoxicity, permeability, and inflammation of metal oxide nanoparticles. Cell Biol Toxicol. 2011;27(5):333–42.
Tolliver LM, Holl NJ, Hou FYS, Lee H-J, Cambre MH, Huang Y-W. Differential cytotoxicity induced by transition metal oxide nanoparticles is a function of cell killing and suppression of cell proliferation. Int J Mol Sci. 2020;21(5):1731.
Rothen-Rutishauser B, Grass RN, Blank F, Limbach LK, Mühlfeld C, Brandenberger C, et al. Direct combination of nanoparticle fabrication and exposure to lung cell cultures in a closed setup as a method to simulate accidental nanoparticle exposure of humans. Environ Sci Technol. 2009;43(7):2634–40.
Benameur L, Auffan M, Cassien M, Liu W, Culcasi M, Rahmouni H, et al. DNA damage and oxidative stress induced by CeO2 nanoparticles in human dermal fibroblasts: Evidence of a clastogenic effect as a mechanism of genotoxicity. Nanotoxicology. 2015;9(6):696–705.
Gojova A, Lee J-T, Jung HS, Guo B, Barakat AI, Kennedy IM. Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells. Inhalation Toxicol. 2009;21(Suppl 1):123–30.
Hildebrand H, Kühnel D, Potthoff A, Mackenzie K, Springer A, Schirmer K. Evaluating the cytotoxicity of palladium/magnetite nano-catalysts intended for wastewater treatment. Environ Pollut (Barking, Essex 1987). 2010;158(1):65–73.
Lai JCK, Lai MB, Jandhyam S, Dukhande VV, Bhushan A, Daniels CK, et al. Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts. Int J Nanomed. 2008;3(4):533–45.
Ahamed M, Siddiqui MA, Akhtar MJ, Ahmad I, Pant AB, Alhadlaq HA. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem Biophys Res Commun. 2010;396(2):578–83.
Vergaro V, Aldieri E, Fenoglio I, Marucco A, Carlucci C, Ciccarella G. Surface reactivity and in vitro toxicity on human bronchial epithelial cells (BEAS-2B) of nanomaterials intermediates of the production of titania-based composites. Toxicol Vitro : Int J Publish Assoc BIBRA. 2016;34:171–8.
Dávila-Grana Á, Diego-González L, González-Fernández Á, Simón-Vázquez R. Synergistic effect of metal oxide nanoparticles on cell viability and activation of MAP Kinases and NFκB. Int J Mol Sci. 2018;19(1):246.
Pierscionek BK, Li Y, Schachar RA, Chen W. The effect of high concentration and exposure duration of nanoceria on human lens epithelial cells. Nanomed Nanotechnol Biol Med. 2012;8(3):383–90.
Rafieepour A, Azari MR, Khodagholi F, Jaktaji JP, Mehrabi Y, Peirovi H. The effect of single and combined exposures to magnetite and polymorphous silicon dioxide nanoparticles on the human A 549 cell line: in vitro study. Environ Sci Pollut Res Int. 2019;26(31):31752–62.
Ickrath P, Wagner M, Scherzad A, Gehrke T, Burghartz M, Hagen R, et al. Time-dependent toxic and genotoxic effects of zinc oxide nanoparticles after long-term and repetitive exposure to human mesenchymal stem cells. Int J Environ Res Public Health. 2017;14(12):1590.
Radeloff K, Radeloff A, Ramos Tirado M, Scherzad A, Hagen R, Kleinsasser NH, et al. Toxicity and Functional Impairment in Human Adipose Tissue-Derived Stromal Cells (hASCs) following long-term exposure to Very Small Iron Oxide Particles (VSOPs). Nanomaterials (Basel, Switzerland). 2020;10(4):741.
Jin M, Li N, Sheng W, Ji X, Liang X, Kong B, et al. Toxicity of different zinc oxide nanomaterials and dose-dependent onset and development of Parkinson’s disease-like symptoms induced by zinc oxide nanorods. Environ Int. 2021;146: 106179.
Kumari M, Singh SP, Chinde S, Rahman MF, Mahboob M, Grover P. Toxicity study of cerium oxide nanoparticles in human neuroblastoma cells. Int J Toxicol. 2014;33(2):86–97.
Fernández-Bertólez N, Costa C, Brandão F, Kiliç G, Duarte JA, Teixeira JP, et al. Toxicological assessment of silica-coated iron oxide nanoparticles in human astrocytes. Food Chem Toxicol : Int J Publish Bri Industr Biol Res Assoc. 2018;118:13–23.
Gliga AR, Di Bucchianico S, Åkerlund E, Karlsson HL. Transcriptome profiling and toxicity following long-term, low dose exposure of human lung cells to Ni and NiO nanoparticles-comparison with NiCl 2. Nanomaterials (Basel, Switzerland). 2020;10(4):649.
Kennedy IM, Wilson D, Barakat AI. Uptake and inflammatory effects of nanoparticles in a human vascular endothelial cell line. Res Rep (Health Effects Institute). 2009;136:3–32.
Schanen BC, Das S, Reilly CM, Warren WL, Self WT, Seal S, et al. Immunomodulation and T helper TH 1 /TH 2 response polarization by CeO 2 and TiO 2 nanoparticles. PLoS ONE. 2013;8(5): e62816.
Seker S, Elçin AE, Yumak T, Sınağ A, Elçin YM. In vitro cytotoxicity of hydrothermally synthesized ZnO nanoparticles on human periodontal ligament fibroblast and mouse dermal fibroblast cells. Toxicol Vitro : Int J Publish Assoc BIBRA. 2014;28(8):1349–58.
Könen-Adıgüzel S, Ergene S. In vitro evaluation of the genotoxicity of CeO 2 nanoparticles in human peripheral blood lymphocytes using cytokinesis-block micronucleus test, comet assay, and gamma H2AX. Toxicol Ind Health. 2018;34(5):293–300.
Henson TE, Navratilova J, Tennant AH, Bradham KD, Rogers KR, Hughes MF. In vitro intestinal toxicity of copper oxide nanoparticles in rat and human cell models. Nanotoxicology. 2019;13(6):795–811.
Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect. 2007;115(3):403–9.
Alarifi S, Ali D, Alkahtani S, Verma A, Ahamed M, Ahmed M, et al. Induction of oxidative stress, DNA damage, and apoptosis in a malignant human skin melanoma cell line after exposure to zinc oxide nanoparticles. Int J Nanomed. 2013;8:983–93.
Åkerlund E, Islam MS, McCarrick S, Alfaro-Moreno E, Karlsson HL. Inflammation and (secondary) genotoxicity of Ni and NiO nanoparticles. Nanotoxicology. 2019;13(8):1060–72.
Jiménez-Chávez A, Solorio-Rodríguez A, Escamilla-Rivera V, Leseman D, Morales-Rubio R, Uribe-Ramírez M, et al. Inflammatory response in human alveolar epithelial cells after TiO 2 NPs or ZnO NPs exposure: Inhibition of surfactant protein A expression as an indicator for loss of lung function. Environ Toxicol Pharmacol. 2021;86: 103654.
Hussain S, Garantziotis S. Interplay between apoptotic and autophagy pathways after exposure to cerium dioxide nanoparticles in human monocytes. Autophagy. 2013;9(1):101–3.
Abudayyak M, Öztaş E, Arici M, Özhan G. Investigation of the toxicity of bismuth oxide nanoparticles in various cell lines. Chemosphere. 2017;169:117–23.
Fahmy HM, Ebrahim NM, Gaber MH. In-vitro evaluation of copper/copper oxide nanoparticles cytotoxicity and genotoxicity in normal and cancer lung cell lines. J Trace Elem Med Biol. 2020;60: 126481.
Božinović K, Nestić D, Centa UG, Ambriović-Ristov A, Dekanić A, de Bisschop L, et al. In-vitro toxicity of molybdenum trioxide nanoparticles on human keratinocytes. Toxicology. 2020;444: 152564.
Ahamed M, Alhadlaq HA, Alam J, Khan MAM, Ali D, Alarafi S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr Pharm Des. 2013;19(37):6681–90.
Pelclova D, Zdimal V, Kacer P, Fenclova Z, Vlckova S, Komarc M, et al. Leukotrienes in exhaled breath condensate and fractional exhaled nitric oxide in workers exposed to TiO2 nanoparticles. J Breath Res. 2016;10(3): 036004.
Valdiglesias V, Costa C, Kiliç G, Costa S, Pásaro E, Laffon B, et al. Neuronal cytotoxicity and genotoxicity induced by zinc oxide nanoparticles. Environ Int. 2013;55:92–100.
Verdon R, Gillies SL, Brown DM, Henry T, Tran L, Tyler CR, et al. Neutrophil activation by nanomaterials in vitro : comparing strengths and limitations of primary human cells with those of an immortalized (HL-60) cell line. Nanotoxicology. 2021;15(1):1–20.
Siddiqui MA, Ahamed M, Ahmad J, Majeed Khan MA, Musarrat J, Al-Khedhairy AA, et al. Nickel oxide nanoparticles induce cytotoxicity, oxidative stress and apoptosis in cultured human cells that is abrogated by the dietary antioxidant curcumin. Food Chem Toxicol : Int J Publish Bri Industr Biol Res Assoc. 2012;50(3–4):641–7.
Park E-J, Choi J, Park Y-K, Park K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology. 2008;245(1–2):90–100.
Fakhar-e-Alam M, Kishwar S, Willander M. Photodynamic effects of zinc oxide nanowires in skin cancer and fibroblast. Lasers Med Sci. 2014;29(3):1189–94.
Hackenberg S, Zimmermann F-Z, Scherzed A, Friehs G, Froelich K, Ginzkey C, et al. Repetitive exposure to zinc oxide nanoparticles induces dna damage in human nasal mucosa mini organ cultures. Environ Mol Mutagen. 2011;52(7):582–9.
Andujar P, Simon-Deckers A, Galateau-Sallé F, Fayard B, Beaune G, Clin B, et al. Role of metal oxide nanoparticles in histopathological changes observed in the lung of welders. Part Fibre Toxicol. 2014;11:23.
Sharma V, Anderson D, Dhawan A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis. 2012;17(8):852–70.
Senapati VA, Kumar A, Gupta GS, Pandey AK, Dhawan A. ZnO nanoparticles induced inflammatory response and genotoxicity in human blood cells: a mechanistic approach. Food Chem Toxicol. 2015;85:61–70.
Vlaanderen J, Pronk A, Rothman N, Hildesheim A, Silverman D, Hosgood HD, et al. A cross-sectional study of changes in markers of immunological effects and lung health due to exposure to multi-walled carbon nanotubes. Nanotoxicology. 2017;11(3):395–404.
Asghar W, Shafiee H, Velasco V, Sah VR, Guo S, El Assal R, et al. Toxicology study of single-walled carbon nanotubes and reduced graphene oxide in human sperm. Sci Rep. 2016;6:30270.
Periasamy VS, Athinarayanan J, Alfawaz MA, Alshatwi AA. Carbon nanoparticle induced cytotoxicity in human mesenchymal stem cells through upregulation of TNF3, NFKBIA and BCL2L1 genes. Chemosphere. 2016;144:275–84.
Beard JD, Erdely A, Dahm MM, de Perio MA, Birch ME, Evans DE, et al. Carbon nanotube and nanofiber exposure and sputum and blood biomarkers of early effect among U.S. workers. Environ Int. 2018;116:214–28.
Zhang W, Zhang D, Tan J, Cong H. Carbon nanotube exposure sensitize human ovarian cancer cells to paclitaxel. J Nanosci Nanotechnol. 2012;12(9):7211–4.
de Gabory L, Bareille R, Daculsi R, L’Azou B, Flahaut E, Bordenave L. Carbon nanotubes have a deleterious effect on the nose: the first in vitro data. Rhinology. 2011;49(4):445–52.
Eldawud R, Wagner A, Dong C, Stueckle TA, Rojanasakul Y, Dinu CZ. Carbon nanotubes physicochemical properties influence the overall cellular behavior and fate. NanoImpact. 2018;9:72–84.
Pacurari M, Qian Y, Fu W, Schwegler-Berry D, Ding M, Castranova V, et al. Cell permeability, migration, and reactive oxygen species induced by multiwalled carbon nanotubes in human microvascular endothelial cells. J Toxicol Environ Health A. 2012;75(2):112–28.
Reamon-Buettner SM, Hackbarth A, Leonhardt A, Braun A, Ziemann C. Cellular senescence as a response to multiwalled carbon nanotube (MWCNT) exposure in human mesothelial cells. Mech Ageing Dev. 2021;193: 111412.
Phuyal S, Kasem M, Knittelfelder O, Sharma A, Fonseca DDM, Vebraite V, et al. Characterization of the proteome and lipidome profiles of human lung cells after low dose and chronic exposure to multiwalled carbon nanotubes. Nanotoxicology. 2018;12(2):138–52.
Witzmann FA, Monteiro-Riviere NA. Multi-walled carbon nanotube exposure alters protein expression in human keratinocytes. Nanomedicine. 2006;2(3):158–68.
Snyder RJ, Hussain S, Rice AB, Garantziotis S. Multiwalled carbon nanotubes induce altered morphology and loss of barrier function in human bronchial epithelium at noncytotoxic doses. Int J Nanomed. 2014;9:4093–105.
Snyder RJ, Verhein KC, Vellers HL, Burkholder AB, Garantziotis S, Kleeberger SR. Multi-walled carbon nanotubes upregulate mitochondrial gene expression and trigger mitochondrial dysfunction in primary human bronchial epithelial cells. Nanotoxicology. 2019;13(10):1344–61.
Yu M, Chen R, Jia Z, Chen J, Lou J, Tang S, et al. MWCNTs Induce ROS Generation, ERK Phosphorylation, and SOD-2 expression in human mesothelial cells. Int J Toxicol. 2016;35(1):17–26.
Rizk MZ, Ali SA, Kadry MO, Fouad GI, Kamel NN, Younis EA, et al. C-reactive protein signaling and chromosomal abnormalities in nanotoxicity induced via different doses of TiO 2 (80 nm) boost liver function. Biol Trace Elem Res. 2020;198(1):157–67.
Jos A, Pichardo S, Puerto M, Sánchez E, Grilo A, Cameán AM. Cytotoxicity of carboxylic acid functionalized single wall carbon nanotubes on the human intestinal cell line Caco-2. Toxicol Vitro : Int J Published Assoc BIBRA. 2009;23(8):1491–6.
Vankoningsloo S, Piret J-P, Saout C, Noel F, Mejia J, Zouboulis CC, et al. Cytotoxicity of multi-walled carbon nanotubes in three skin cellular models: effects of sonication, dispersive agents and corneous layer of reconstructed epidermis. Nanotoxicology. 2010;4(1):84–97.
Herzog E, Byrne HJ, Davoren M, Casey A, Duschl A, Oostingh GJ. Dispersion medium modulates oxidative stress response of human lung epithelial cells upon exposure to carbon nanomaterial samples. Toxicol Appl Pharmacol. 2009;236(3):276–81.
Müller L, Riediker M, Wick P, Mohr M, Gehr P, Rothen-Rutishauser B. Oxidative stress and inflammation response after nanoparticle exposure: differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J R Soc Interface. 2010;7(Suppl 1):S27–40.
PubMed Google Scholar
Basak SC, Vracko M, Witzmann FA. Mathematical nanotoxicoproteomics: quantitative characterization of effects of Multi-walled Carbon Nanotubes (MWCNT) and TiO2 Nanobelts (TiO2-NB) on protein expression patterns in human intestinal cells. Curr Comput Aided Drug Des. 2016;12(4):259–64.
Patlolla A, Patlolla B, Tchounwou P. Evaluation of cell viability, DNA damage, and cell death in normal human dermal fibroblast cells induced by functionalized multiwalled carbon nanotube. Mol Cell Biochem. 2010;338(1–2):225–32.
Dahm MM, Schubauer-Berigan MK, Evans DE, Birch ME, Bertke S, Beard JD, et al. Exposure assessments for a cross-sectional epidemiologic study of US carbon nanotube and nanofiber workers. Int J Hyg Environ Health. 2018;221(3):429–40.
Fatkhutdinova LM, Khaliullin TO, Vasil’yeva OL, Zalyalov RR, Mustafin IG, Kisin ER, et al. Fibrosis biomarkers in workers exposed to MWCNTs. Toxicol Appl Pharmacol. 2016;299:125–31.
Zhao X, Chang S, Long J, Li J, Li X, Cao Y. The toxicity of multi-walled carbon nanotubes (MWCNTs) to human endothelial cells: The influence of diameters of MWCNTs. Food Chem Toxicol : Int J Publish Bri Industr Biol Res Assoc. 2019;126:169–77.
Öner D, Ghosh M, Coorens R, Bové H, Moisse M, Lambrechts D, et al. Induction and recovery of CpG site specific methylation changes in human bronchial cells after long-term exposure to carbon nanotubes and asbestos. Environ Int. 2020;137: 105530.
Luanpitpong S, Wang L, Castranova V, Rojanasakul Y. Induction of stem-like cells with malignant properties by chronic exposure of human lung epithelial cells to single-walled carbon nanotubes. Part Fibre Toxicol. 2014;11:22.
Shvedova AA, Yanamala N, Kisin ER, Khailullin TO, Birch ME, Fatkhutdinova LM. Integrated analysis of dysregulated ncRNA and mRNA expression profiles in humans exposed to carbon nanotubes. PLoS ONE. 2016;11(3): e0150628.
Domenech J, Hernández A, Demir E, Marcos R, Cortés C. Interactions of graphene oxide and graphene nanoplatelets with the in vitro Caco-2/HT29 model of intestinal barrier. Sci Rep. 2020;10(1):2793.
Wang L, Stueckle TA, Mishra A, Derk R, Meighan T, Castranova V, et al. Neoplastic-like transformation effect of single-walled and multi-walled carbon nanotubes compared to asbestos on human lung small airway epithelial cells. Nanotoxicology. 2014;8(5):485–507.
Mukherjee SP, Gupta G, Klöditz K, Wang J, Rodrigues AF, Kostarelos K, et al. Next-generation sequencing reveals differential responses to acute versus long-term exposures to graphene oxide in human lung cells. Small. 2020;16(21): e1907686.
Pérez RF, Soto Fernández AY, Bousquets Muñoz P, Sierra MI, Tejedor JR, Morales-Sánchez P, et al. No genome-wide DNA methylation changes found associated with medium-term reduced graphene oxide exposure in human lung epithelial cells. Epigenetics. 2020;15(3):283–93.
Xu Y, Luo Z, Li S, Li W, Zhang X, Zuo YY, et al. Perturbation of the pulmonary surfactant monolayer by single-walled carbon nanotubes: a molecular dynamics study. Nanoscale. 2017;9(29):10193–204.
Gasser M, Wick P, Clift MJD, Blank F, Diener L, Yan B, et al. Pulmonary surfactant coating of multi-walled carbon nanotubes (MWCNTs) influences their oxidative and pro-inflammatory potential in vitro. Part Fibre Toxicol. 2012;9:17.
Di Cristo L, Grimaldi B, Catelani T, Vázquez E, Pompa PP, Sabella S. Repeated exposure to aerosolized graphene oxide mediates autophagy inhibition and inflammation in a three-dimensional human airway model. Materials Today Bio. 2020;6: 100050.
Chortarea S, Clift MJD, Vanhecke D, Endes C, Wick P, Petri-Fink A, et al. Repeated exposure to carbon nanotube-based aerosols does not affect the functional properties of a 3D human epithelial airway model. Nanotoxicology. 2015;9(8):983–93.
Sargent JF. Nanotechnology: a policy primer. Congres Res Serv. 2011;4(3/4):191–203.
Duschl A, Windgasse G. A survey on the state of nanosafety research in the European Union and the United States. J Nanoparticles Res. 2018;20(12):335.
UNECA. Towards African nanotechnology future: trends, impacts and opportunities. Addis Ababa: United Nations. Economic Commission for Africa; 2020. Available from: https://archive.uneca.org/publications/towards-african-nanotechnology-future-trendsimpacts-and-opportunities .
Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules. 2019;25(1):112.
Huang Q, Yu H, Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci. 2010;75(1):R50–7.
Maiti D, Tong X, Mou X, Yang K. Carbon-based nanomaterials for biomedical applications: a recent study. Front Pharmacol. 2019;9:1401.
Pandey P, Dahiya M. A brief review on inorganic nanoparticles. J Crit Rev. 2016;3(3):18.
Lohse SE, Murphy CJ. Applications of colloidal inorganic nanoparticles: from medicine to energy. J Am Chem Soc. 2012;134(38):15607–20.
Wang P, Lombi E, Zhao FJ, Kopittke PM. Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci. 2016;21(8):699–712.
Bahadar H, Maqbool F, Niaz K, Abdollahi M. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed J. 2016;20(1):1–11.
PubMed PubMed Central Google Scholar
Baktur R, Patel H, Kwon S. Effect of exposure conditions on SWCNT-induced inflammatory response in human alveolar epithelial cells. Toxicol Vitro : Int J Published Assoc BIBRA. 2011;25(5):1153–60.
Liu G, Gao J, Ai H, Chen X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small. 2013;9–10:1533–45.
Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7:193.
Dadwal A, Baldi A, Narang RK. Nanoparticles as carriers for drug delivery in cancer. Artificial Cells, Nanomed Biotechnol. 2018;46(Sup2):295–305.
Kermanizadeh A, Lohr M, Roursgaard M, Messner S, Gunness P, Kelm JM, et al. Hepatic toxicology following single and multiple exposure of engineered nanomaterials utilising a novel primary human 3D liver microtissue model. Part Fibre Toxicol. 2014;11:56.
Joris F, Valdepérez D, Pelaz B, Soenen SJ, Manshian BB, Parak WJ, et al. The impact of species and cell type on the nanosafety profile of iron oxide nanoparticles in neural cells. J Nanobiotechnol. 2016;14:69.
Wang Y, Chen S, Yan Z, Pei M. A prospect of cell immortalization combined with matrix microenvironmental optimization strategy for tissue engineering and regeneration. Cell Biosci. 2019;9:7.
Zhang S, Zhang X, Liu H, Qu W, Guan Z, Zeng Q, et al. Modifying effect of COMT gene polymorphism and a predictive role for proteomics analysis in children’s intelligence in endemic fluorosis area in Tianjin, China. Toxicol Sci J Soc Toxicol. 2015;144(2):238–45.
Mukherjee SG, O’Claonadh N, Casey A, Chambers G. Comparative in vitro cytotoxicity study of silver nanoparticle on two mammalian cell lines. Toxicol In Vitro. 2012;26(2):238–51.
Ekstrand-Hammarström B, Akfur CM, Andersson PO, Lejon C, Österlund L, Bucht A. Human primary bronchial epithelial cells respond differently to titanium dioxide nanoparticles than the lung epithelial cell lines A549 and BEAS-2B. Nanotoxicology. 2011;6(6):623–34.
Kermanizadeh A, Pojana G, Gaiser BK, Birkedal R, Bilanicová D, Wallin H, et al. In vitro assessment of engineered nanomaterials using a hepatocyte cell line: cytotoxicity, pro-inflammatory cytokines and functional markers. Nanotoxicology. 2013;7(3):301–13.
Bundschuh M, Filser J, Lüderwald S, McKee MS, Metreveli G, Schaumann GE, et al. Nanoparticles in the environment: where do we come from, where do we go to? Environ Sci Eur. 2018;30(1):6.
Tolaymat T, Genaidy A, Abdelraheem W, Dionysiou D, Andersen C. The effects of metallic engineered nanoparticles upon plant systems: An analytic examination of scientific evidence. Sci Total Environ. 2017;579(1):93–106.
Keller AA, McFerran S, Lazareva A, Suh S. Global life cycle releases of engineered nanomaterials. J Nanopart Res. 2013;15:1692.
Gottschalka F, Nowack B. The release of engineered nanomaterials to the environment. J Environ Monitor. 2011;13:1145–55.
Download references
Acknowledgements
We would like to thank Daphne Silva Pino and Romana Petry for their contribution at the initial stages of this research.
This research was funded through the GRUN project (Towards the first implementation of groundwater remediation using nanotechnology in Brazil) as part of UKRI’s Global Challenges Research Fund (GCRF) and a grant from the São Paulo Research Foundation (FAPESP) (Grant 17/20308–0).
Author information
Authors and affiliations.
Depeartment of International Public Health, Liverpool School of Tropical Medicine, Liverpool, UK
Elizabeth Adjoa Kumah
School of Computing, Engineering & Digital Technologies, Teesside University, Middlesbrough, TS1 3BX, UK
Raoul Djou Fopa, Saeed Harati & Tannaz Pak
Department of Health Services Research and Policy, London School of Hygiene and Tropical Medicine, London, UK
School of Health and Life Sciences, Teesside University, Middlesbrough, UK
Fatemeh Vida Zohoori
You can also search for this author in PubMed Google Scholar
Contributions
Elizabeth Adjoa Kumah: Conceptualization, Methodology, Investigation, Formal analysis, Validation, Supervision, Writing—Original Draft, Writing—Review & Editing, Raoul Djou Fopa: Investigation, Data Curation, Saeed Harati: Investigation, Data Curation, Paul Boadu: Investigation, Data Curation, Formal analysis, Fatemeh Vida Zohoori: Conceptualization, Methodology, Validation, Supervision, Writing—Review & Editing, Funding acquisition, Tannaz Pak: Conceptualization, Methodology, Validation, Supervision, Writing—Review & Editing, Funding acquisition. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Tannaz Pak .
Ethics declarations
Ethics approval and consent to participate.
The research presented in this article is a scoping review, as such, no ethical approval was needed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
MEDLINE Search History.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kumah, E.A., Fopa, R.D., Harati, S. et al. Human and environmental impacts of nanoparticles: a scoping review of the current literature. BMC Public Health 23 , 1059 (2023). https://doi.org/10.1186/s12889-023-15958-4
Download citation
Received : 15 August 2022
Accepted : 22 May 2023
Published : 03 June 2023
DOI : https://doi.org/10.1186/s12889-023-15958-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cytotoxicity; Environmental health
- Genotoxicity
- Human health
- Nanoparticles
- Scoping review
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 07 June 2022
Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists
- Nadeem Joudeh 1 &
- Dirk Linke 1
Journal of Nanobiotechnology volume 20 , Article number: 262 ( 2022 ) Cite this article
81k Accesses
297 Citations
4 Altmetric
Metrics details
Interest in nanomaterials and especially nanoparticles has exploded in the past decades primarily due to their novel or enhanced physical and chemical properties compared to bulk material. These extraordinary properties have created a multitude of innovative applications in the fields of medicine and pharma, electronics, agriculture, chemical catalysis, food industry, and many others. More recently, nanoparticles are also being synthesized ‘biologically’ through the use of plant- or microorganism-mediated processes, as an environmentally friendly alternative to the expensive, energy-intensive, and potentially toxic physical and chemical synthesis methods. This transdisciplinary approach to nanoparticle synthesis requires that biologists and biotechnologists understand and learn to use the complex methodology needed to properly characterize these processes. This review targets a bio-oriented audience and summarizes the physico–chemical properties of nanoparticles, and methods used for their characterization. It highlights why nanomaterials are different compared to micro- or bulk materials. We try to provide a comprehensive overview of the different classes of nanoparticles and their novel or enhanced physicochemical properties including mechanical, thermal, magnetic, electronic, optical, and catalytic properties. A comprehensive list of the common methods and techniques used for the characterization and analysis of these properties is presented together with a large list of examples for biogenic nanoparticles that have been previously synthesized and characterized, including their application in the fields of medicine, electronics, agriculture, and food production. We hope that this makes the many different methods more accessible to the readers, and to help with identifying the proper methodology for any given nanoscience problem.
Nano etymology
The prefix nano is derived from the Greek word nanos, “a dwarf”. In 1947, at the 14th conference of the International Union of Pure and Applied Chemistry (IUPAC), the prefix nano was officially adopted to describe the one-billionth part (10 –9 ) of a unit Footnote 1 . In scientific literature, the prefix nano has been adopted as a popular label in many fields of modern science to describe small entities and processes. These terms include, but are not limited to nanoscience, nanotechnology, nanorobots, nanomagnets, nanoelectronics, nanoencapsulation, etc. [ 1 ]. In all of these cases, the prefix nano is used to describe “very small” entities or processes, most often at actual nanometer scale.
Definitions
Nanoscience is a branch of science that comprises the study of properties of matter at the nanoscale, and particularly focuses on the unique, size-dependent properties of solid-state materials [ 2 ]. Nanotechnology is the branch that comprises the synthesis, engineering, and utilization of materials whose size ranges from 1 to 100 nm, known as nanomaterials [ 3 ]. The birth of nanoscience and nanotechnology concepts is usually linked to the famous lecture of Nobel laureate Richard Feynman at the 1959 meeting of the American Physical Society, ‘‘There’s Plenty of Room at the Bottom’’ [ 4 ]. However, the use of nanotechnology and nanomaterials goes back in history long before that.
History of nanotechnology
Long before the era of nanotechnology, people were unknowingly coming across various nanosized objects and using nano-level processes. In ancient Egypt, dyeing hair in black was common and was for a long time believed to be based on plant products such as henna [ 5 ]. However, recent research on hair samples from ancient Egyptian burial sites showed that hair was dyed with paste from lime, lead oxide, and water [ 6 ]. In this dyeing process, galenite (lead sulfide, PbS) nanoparticles are formed. The ancient Egyptians were able to make the dyeing paste react with sulfur (part of hair keratin) and produce small PbS nanoparticles which provided even and steady dyeing.
Probably the most famous example for the ancient use of nanotechnology is the Lycurgus Cup (fourth century CE). This ancient roman cup possesses unusual optical properties; it changes its color based on the location of the light source. In natural light, the cup is green, but when it is illuminated from within (with a candle), it becomes red. The recent analysis of this cup showed that it contains 50–100 nm Au and Ag nanoparticles [ 7 ], which are responsible for the unusual coloring of the cup through the effects of plasmon excitation of electrons [ 8 ]. The ancient use of nanotechnology does not stop here, in fact, there is evidence for the early use of nanotechnology processes in Mesopotamia, Ancient India, and the Maya [ 9 , 10 ].
Why nanomaterials are different
Today, due to their unique properties, nanomaterials are used in a wide range of applications, such as catalysis, water treatment, energy storage, medicine, agriculture, etc . [ 11 , 12 , 13 ]. Two main factors cause nanomaterials to behave significantly differently than the same materials at larger dimensions: surface effects and quantum effects [ 14 ]. These factors make nanomaterials exhibit enhanced or novel mechanical, thermal, magnetic, electronic, optical, and catalytic properties [ 1 , 15 , 16 ].
Nanomaterials have different surface effects compared to micromaterials or bulk materials, mainly due to three reasons; (a) dispersed nanomaterials have a very large surface area and high particle number per mass unit, (b) the fraction of atoms at the surface in nanomaterials is increased, and (c) the atoms situated at the surface in nanomaterials have fewer direct neighbors [ 1 , 14 ]. As a consequence of each of these differences, the chemical and physical properties of nanomaterials change compared to their larger-dimension counterparts. For instance, having fewer direct neighbor atoms for the atoms situated at the surface results in lowering the binding energy per atom for nanomaterials. This change directly affects the melting temperature of nanomaterials following the Gibbs–Thomson equation, e.g., the melting point of 2.5 nm gold nanoparticles is 407 degrees lower than the melting point of bulk gold [ 14 ]. Larger surface areas and larger surface-to-volume ratios generally increases the reactivity of nanomaterials due to the larger reaction surface [ 1 ], as well as resulting in significant effects of surface properties on their structure [ 17 ]. The dispersity of nanomaterials is a key factor for the surface effects. The strong attractive interactions between particles can result in the agglomeration and aggregation of nanomaterials, which negatively affects their surface area and their nanoscale properties [ 18 ]. Agglomeration can be prevented by increasing the zeta potential of nanomaterials (increasing the repulsive force) [ 19 ], optimizing the degree of hydrophilicity/hydrophobicity of the nanomaterial, or by optimizing the pH and the ionic strength of the suspension medium [ 20 ].
Nanomaterials display distinct size-dependent properties in the 1–100 nm range where quantum phenomena are involved. When the material radius approaches the asymptotic exciton Bohr radius (the separation distance between the electron and hole), the influence of quantum confinement becomes apparent [ 17 ]. In other words, by shrinking the size of the material, quantum effects become more pronounced, and nanomaterials become quantal. Those quantum structures are physical structures where all the charge carriers (electrons and holes) are confined within the physical dimensions [ 21 ]. As a result of quantum confinement effects, for instance, some non-magnetic materials in bulk such as palladium, platinum, and gold become magnetic in the nanoscale [ 14 ]. Quantum confinement can also result in significant changes in electron affinity or the ability to accept or donate electrical charges, which is directly reflected on the catalytic properties of the material. For example, the catalytic activity of cationic platinum clusters in N 2 O decomposition is dictated by the number of atoms in the cluster. 6–9, 11, 12, 15, and 20 atom-containing clusters are very reactive, while clusters with 10, 13, 14, and 19 atoms have low reactivity [ 14 ].
Classification of nanomaterials
The key elements of nanotechnology are the nanomaterials. Nanomaterials are defined as materials where at least one of their dimensions is in the nanoscale, i.e. smaller than 100 nm [ 22 ]. Based on their dimensionalities, nanomaterials are placed into four different classes, summarized in Fig. 1 .
Zero-dimensional nanomaterials (0-D): the nanomaterials in this class have all their three dimensions in the nanoscale range. Examples are quantum dots, fullerenes, and nanoparticles.
One-dimensional nanomaterials (1-D): the nanomaterials in this class have one dimension outside the nanoscale. Examples are nanotubes, nanofibers, nanorods, nanowires, and nanohorns.
Two-dimensional nanomaterials (2-D): the nanomaterials in this class have two dimensions outside the nanoscale. Examples are nanosheets, nanofilms, and nanolayers.
Three-dimensional nanomaterials (3-D) or bulk nanomaterials: in this class the materials are not confined to the nanoscale in any dimension. This class contains bulk powders, dispersions of nanoparticles, arrays of nanowires and nanotubes, etc .
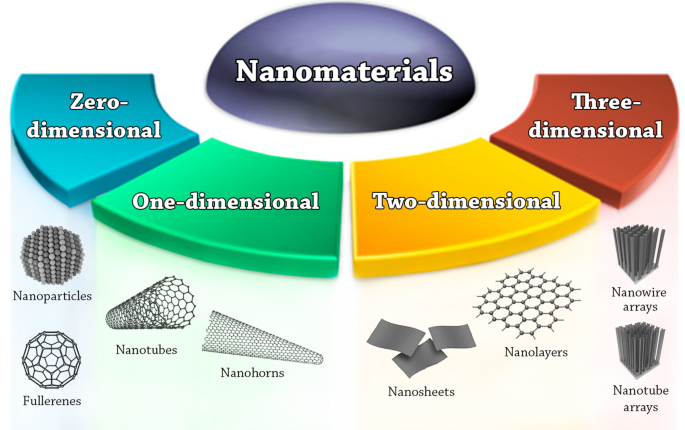
Nanomaterials classification based on dimensionality
Nanoparticles (NPs)
The International Organization for Standardization (ISO) defines nanoparticles as nano-objects with all external dimensions in the nanoscale, where the lengths of the longest and the shortest axes of the nano-object do not differ significantly. If the dimensions differ significantly (typically by more than three times), terms such as nanofibers or nanoplates maybe preferred to the term NPs Footnote 2 .
NPs can be of different shapes, sizes, and structures. They can be spherical, cylindrical, conical, tubular, hollow core, spiral, etc., or irregular [ 23 ]. The size of NPs can be anywhere from 1 to 100 nm. If the size of NPs gets lower than 1 nm, the term atom clusters is usually preferred. NPs can be crystalline with single or multi-crystal solids, or amorphous. NPs can be either loose or agglomerated [ 24 ].
NPs can be uniform, or can be composed of several layers. In the latter case, the layers often are: (a) The surface layer, which usually consists of a variety of small molecules, metal ions, surfactants, or polymers. (b) The shell layer, which is made of a chemically different material from the core layer. (c) The core layer, which is the central portion of the NP [ 25 ].
Classification of NPs
Based on their composition, NPs are generally placed into three classes: organic, carbon-based, and inorganic [ 23 ].
Organic NPs
This class comprises NPs that are made of proteins, carbohydrates, lipids, polymers, or any other organic compounds [ 26 ]. The most prominent examples of this class are dendrimers, liposomes, micelles, and protein complexes such as ferritin (shown in Fig. 2 ). These NPs are typically non-toxic, bio-degradable, and can in some cases, e.g., for liposomes, have a hollow core. Organic NPs are sensitive to thermal and electromagnetic radiation such as heat and light [ 23 ]. In addition, they are often formed by non-covalent intermolecular interactions, which makes them more labile in nature and offers a route for clearance from the body [ 27 ]. There are different parameters that determine the potential field of application of organic NPs, e.g., composition, surface morphology, stability, carrying capacity, etc . Today, organic NPs are mostly used in the biomedical field in targeted drug delivery [ 23 ] and cancer therapy [ 28 ].
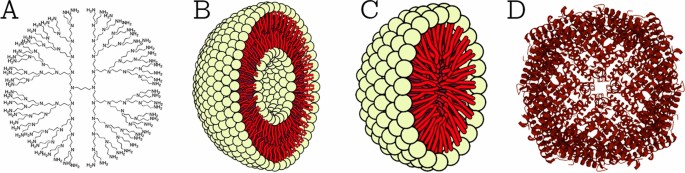
Types of organic NPs. A Dendrimers; B liposomes; C micelles; and D ferritin
Carbon-based NPs
This class comprises NPs that are made solely from carbon atoms [ 23 ]. Famous examples of this class are fullerenes, carbon black NPs, and carbon quantum dots (shown in Fig. 3 ). Fullerenes are carbon molecules that are characterized by a symmetrical closed-cage structure. C 60 fullerenes consist of 60 carbon atoms arranged in the shape of a soccer ball [ 29 ], but also other types of fullerenes such as C 70 and C 540 fullerenes have been described [ 30 ]. Carbon black NPs are grape-like aggregates of highly fused spherical particles [ 31 ]. Carbon quantum dots consist of discrete, quasi-spherical carbon NPs with sizes below 10 nm [ 32 ]. Carbon-based NPs unite the distinctive properties of sp 2 -hybridized carbon bonds with the unusual physicochemical properties at the nanoscale. Due to their unique electrical conductivity, high strength, electron affinity, optical, thermal, and sorption properties [ 25 , 33 ], carbon-based NPs are used in a wide range of application such as drug delivery [ 34 ], energy storage [ 35 ], bioimaging [ 36 ], photovoltaic devices, and environmental sensing applications to monitor microbial ecology or to detect microbial pathogens [ 33 ]. Nanodiamonds and carbon nano onions are more complex, carbon-based NPs. Due to their characteristic low toxicity and biocompatibility, they are used in drug delivery and tissue engineering applications [ 37 , 38 ].
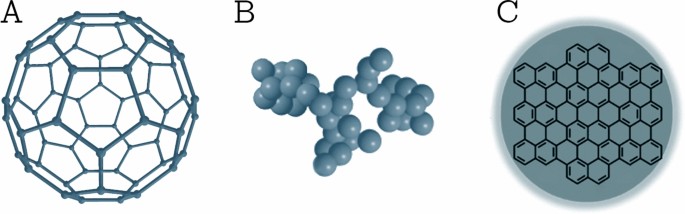
Different types of carbon-based NPs. A C 60 fullerene; B carbon black NPs; and C carbon quantum dots
Inorganic NPs
This class comprises NPs that not made of carbon or organic materials. The typical examples of this class are metal, ceramic, and semiconductor NPs. Metal NPs are purely made of metal precursors, they can be monometallic, bimetallic [ 39 ], or polymetallic [ 40 ]. Bimetallic NPs can be made from alloys or formed in different layers (core–shell) [ 39 ]. Due to the localized surface plasmon resonance characteristics, these NPs possess unique optical and electricals properties [ 25 ]. In addition, some metal NPs also possess unique thermal, magnetic, and biological properties [ 23 ]. This makes them increasingly important materials for the development of nanodevices that can be used in numerous physical, chemical, biological, biomedical, and pharmaceutical applications [ 41 , 42 ] (these applications are discussed in detail later in the applications section of the review). In present days, the size-, shape-, and facet-controlled synthesis of metal NPs is important for creating cutting-edge materials [ 43 ].
Semiconductor NPs are made of semiconductor materials, which possess properties between metals and non-metals. These NPs possess unique wide bandgaps and show significant alteration in their properties with bandgap tuning compared to bulk semiconductor materials [ 25 ]. As a result, these NPs are important materials in photocatalysis, optic, and electronic devices [ 44 , 45 ]. Ceramic NPs are inorganic solids made of carbonates, carbides, phosphates, and oxides of metals and metalloids, such as titanium and calcium [ 46 ]. They are usually synthesized via heat and successive cooling and they can be found in amorphous, polycrystalline, dense, porous or hollow forms [ 25 ]. They are mainly used in biomedical applications due to their high stability and high load capacity [ 47 ]. Nevertheless, they are also used in other applications such as catalysis, degradation of dyes, photonics and optoelectronics [ 46 , 48 ].
Physicochemical properties of NPs
As mentioned earlier, NPs can be used in a long list of applications due to their unique physical and chemical properties that do not exist in their larger-dimension counterparts of the same materials. The following sections summarize the most import physicochemical properties that are changing on the nanoscale.
Mechanical properties
Mechanical properties refer to the mechanical characteristics of a material under different conditions, environments, and various external forces. As for traditional materials, the mechanical properties of nanomaterials generally consist of ten parts: strength, brittleness, hardness, toughness, fatigue strength, plasticity, elasticity, ductility, rigidity, and yield stress [ 49 ]. Most inorganic, non-metallic materials are brittle materials and do not have significant toughness, plasticity, elasticity, or ductility properties. Organic materials on the other hand, are flexible materials and do not necessarily have brittleness and rigidity properties.
Due to surface and quantum effects, NPs display different mechanical properties compared to bulk materials [ 49 ]. For example, conventional FeAl powder which is composed of microparticles (larger than 4 µm), is brittle, while ultrafine FeAl alloy powder displays a good combination of strength and ductility as well as enhanced plasticity [ 50 ]. These new properties are believed to arise due to the diverse interaction forces between NPs or between them and a surface. The most important interaction forces involved are van der Waals forces, which consist of three parts, Keesom force, Debye force, and London force [ 51 , 52 , 53 ]. Other relevant interaction forces are electrostatic and electrical double layer forces, normal and lateral capillary forces, solvation, structural, and hydration forces [ 54 ].
There are different theories on how the interaction forces between NPs give them new mechanical properties, such as the DLVO (Derjaguin–Landau–Verwey–Overbeek) theory, JKR (Johnson–Kendall–Roberts) theory, and DMT (Derjaguin–Muller–Toporov) theory. The DLVO theory combines the effects of van der Waals attraction and electrostatic repulsion to describe the stability of colloidal dispersions [ 54 ]. This theory can explain many phenomena in colloidal science, such as the adsorption and the aggregation of NPs in aqueous solutions and the force between charged surfaces interacting through a liquid medium [ 55 , 56 ]. Nevertheless, the DLVO theory is inadequate for the colloidal properties in the aggregated state [ 54 ].
When the size of objects decreases to the nanoscale, the surface forces become a major player in their adhesion, contact, and deformation behaviors. The JRK theory is applicable to easily deformable, large bodies with high surface energies, where it describes the domination of surface interactions by strong, short-range adhesion forces. In contrast to this, the DMT theory is applicable to very small and hard bodies with low surface energies, where it describes the adhesion being caused by the presence of weak, long-range attractive forces. Although the DLVO, JKR and DMT theories have been widely used to describe and study the mechanical properties of NPs [ 57 , 58 ], it is still a matter of debate whether or not continuum mechanics can be used to describe a particle or collection of particles at the nanometer scale [ 54 ].
Thermal properties
Heat transfer in NPs primarily depends on energy conduction due to electrons as well as photons (lattice vibration) and the scattering effects that accompany both [ 59 ]. The major components of thermal properties of a material are thermal conductivity, thermoelectric power, heat capacity, and thermal stability [ 59 , 60 ].
NP size has a direct impact on electrical and thermal conductivity of NPs [ 60 ]. As the NP size decreases, the ratio of particle surface area respective to its volume increases hyperbolically [ 60 ]. Since the conduction of electrons is one of the two main ways in which heat is transferred, the higher surface-to-volume ratio in NPs provides higher number of electrons for heat transfer compared to bulk materials [ 61 ]. Moreover, thermal conductivity in NPs is also promoted by microconvection, which results from the Brownian motion of NPs [ 62 ]. Nevertheless, this phenomenon only happens when solid NPs are dispersed in a liquid (generating a Nanofluid) [ 63 ]. As an example, the addition of Cu NPs to ethylene glycol enhances the thermal conductivity of the fluid up to 40% [ 64 ].
The thermoelectric power of a material depends on its Seebeck coefficient and electrical conductivity ( \(P={S}^{2}\sigma \) , where P is thermoelectric power, S is the Seebeck coefficient, and \(\sigma \) is the electrical conductivity). The scattering of NPs in bulk materials (doping) is known to enhance the thermoelectric power factor [ 65 ]. This enhancement could come from the enhancement of the Seebeck coefficient or the enhancement of electrical conductivity. The embedding of size-controlled NPs in bulk thermoelectric materials helps to reduce the lattice thermal conductivity and enhances the Seebeck coefficient due to electron energy filtering [ 66 , 67 ]. Generally, the enhancement of electrical conductivity is accompanied by the reduction of the Seebeck coefficient and vice versa [ 65 ] However, the doping of InGaAlAs material with 2–3 nm Er NPs resulted in the significant increase of thermoelectric power of the material through the enhancement of the conductivity while keeping the Seebeck coefficient unchanged [ 65 ]. Depending on NP size, volume fraction, and band offset, a NP-doped sample can either enhance or suppress the electrical conductivity in comparison with undoped bulk sample.
Experimental studies have shown that the heat capacity of NPs exceeds the values of analogous bulk materials by up to 10% [ 68 ], e.g. in the case of Al 2 O 3 and SiO 2 NPs [ 69 , 70 ]. The major contribution to heat capacity at ambient temperatures is determined by the vibration degrees of freedom, i.e., the peculiarities of phonon spectra (vibrational energy that arises from oscillating atoms within a crystal) are responsible for the anomalous behavior of heat capacity of NPs [ 68 ]. NPs usually exhibit a significant decrease in melting temperature compared to their analogous bulk materials [ 71 ]. The main reason for this phenomenon is that the liquid/vapor interface energy is generally lower than the average solid/vapor interface energy [ 72 ]. When the particle size decreases, its surface-to-volume ratio increases, and the melting temperature decreases as a result of the improved free energy at the particle surface [ 73 ]. For instance, the melting temperature of 3 nm Au NPs is 300 degrees lower than the melting temperature of bulk gold [ 14 ]. In addition, NP composition plays an important role in thermal stability. For example, the thermal stability of Au in Au 0.8 Fe 0.2 is significantly higher than of pure Au or Au 0.2 Fe 0.8 [ 74 ]. Generally, bimetallic alloy NPs show higher thermal stabilities and melting temperatures than monometallic NPs due to the alloying effect [ 75 , 76 ].
Magnetic properties
All magnetic compounds include a ‘magnetic element’ in their formula, i.e., Fe, Co, or Ni (at ambient temperatures). There are only three known exceptions that are made from mixed diamagnetic elements, Sc 3 In, ZrZn 2 , and TiBe 2-x Cu x [ 77 , 78 , 79 , 80 ]. Otherwise, elements such as Pd, Au, or Ag are diamagnetic. This all changes in the nanoscale. Several materials become magnetic in the form of NPs as a result of uneven electronic distribution [ 25 ]. For instance, FeAl is not magnetic in bulk but in the form of NPs, it is becomes magnetic [ 50 ], other examples include Pd and Au [ 81 ]. In bulk materials, the key parameters for determining magnetic properties are composition, crystallographic structure, magnetic anisotropy, and vacancy defects [ 82 , 83 ]. However, on the nanoscale, two more important parameters are strongly involved, i.e., size and shape [ 84 ].
One of the interesting size-dependent phenomena of NPs is superparamagnetism [ 84 ]. As the size of the NPs decreases, the magnetic anisotropy energy per NP decreases. The magnetic anisotropy energy is the energy keeping the magnetic moment in a particular orientation. At a characteristic size for each type of NPs, the anisotropy energy becomes equal to the thermal energy, which allows the random flipping of the magnetic moment [ 85 ], in this case, the NP is defined as being superparamagnetic [ 86 ]. Superparamagnetic NPs display high magnetization only in the presence of a magnetic field, and once it is removed they do not retain any magnetization [ 87 ]. Superparamagnetism was long believed to form only in small ferromagnetic or ferrimagnetic NPs [ 88 ], but interestingly, other paramagnetic materials show magnetism in the nanoscale too [ 81 ].
NP size effects can also be observed in changes in magnetic coercivity, i.e., the resistance of a magnetic material to changes in magnetization (Fig. 4 ). In contrast to large particles or bulk materials, which possess multiple magnetic domain structures, small NPs possess single magnetic domain structures below a certain critical radius (r c ), where all magnetic spins in the NP align unidirectionally (blue arrows in Fig. 4 ). However, the NP radius has to be lower than the threshold radius for superparamagnetism (r sp ) in order to be superparamagnetic [ 89 ]. In the single-domain regime, between r sp and r c , the magnetic coercivity increases as the size of the NP increases until it reaches the maximum at r c [ 84 ]. In this size regime, due to the high magnetic coercivity, the NPs behave similarly as their larger dimension counterparts despite having a single domain structure, i.e., they become ferromagnetic for ferromagnetic materials or paramagnetic for paramagnetic materials etc . Above r c , the magnetic coercivity starts to decrease when multiple magnetic domains are formed in a single NP. The critical radius represents the size where it is energetically favored for the NP to exist without a domain wall [ 86 ]. The calculated critical radii for some common magnetic materials are 35 nm of Ni, 8 nm for Co, and 1 nm for Fe [ 90 ]. Above that point, multi-domain magnetism begins in which a smaller reversal magnetic field is required to make the net magnetization zero [ 84 ].
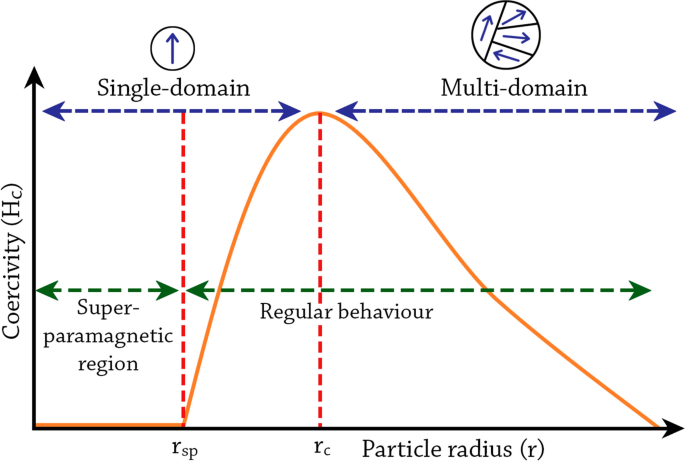
The change in magnetic coercivity of NPs as a function of particle radius. Figure adapted from Kalubowilage et al., 2019 [ 89 ]. rc critical radius, rsp threshold radius for superparamagnetism
The second key parameter for determining the magnetic properties of NPs is the shape of NPs. In comparison to the size parameter, there is significant less research on the effect of shape on the magnetic properties of NPs having the same volume [ 86 ]. However, large differences in coercivity were found between a set of cubic and spherical CoFe 2 O 4 NPs [ 91 ]. Unlike the curved topography in spherical CoFe 2 O 4 NPs, cubic CoFe 2 O 4 NPs have fewer missing oxygen atoms, and it was hypothesized that this led to less surface pinning and to lower coercivity for the cubic structures [ 86 ]. Other studies also found differences in magnetism between spherical and cubic Fe 3 O 4 NPs [ 92 , 93 ].
Similar to bulk materials, the composition also affects the magnetism of NPs. The magnetocrystalline phase of the NP is significant in determining its magnetic coercivity [ 94 ]. This effect can be observed in magnetic bimetallic core–shell or alloy NPs with anisotropic crystalline structures. For example, Co@Pt core–shell NPs composed of an isotropically structured face-centered cubic Co core and a non-magnetic Pt shell exhibit superparamagnetic behavior with zero coercivity at room temperature [ 95 ]. In general, the compositional modification of NPs by the adoption of magnetic dopants is known to significantly change the magnetism of NPs [ 96 ].
Electronic and optical properties
Metallic and semiconductor NPs possess interesting linear absorption, photoluminescence emission, and nonlinear optical properties due to the quantum confinement and localized surface plasmon resonance (LSPR) effect [ 97 , 98 ]. LSPR phenomena arise when the incident photon frequency is constant with the collective excitation of the conductive electrons [ 25 ].Due to this phenomenon, noble metal NPs exhibit a strong size-dependent UV–visible extinction band that is not present in the spectra of bulk metals. Generally, the optical properties of NPs depend on the size, shape, and the dielectric environment of the NPs [ 99 ].
The collective excitations of conductive electrons in metals are called plasmons [ 100 ]. Depending on the boundary conditions, bulk plasmons, surface-propagating plasmons, and surface-localized plasmons are distinguished (Fig. 5 A–C). Because of their longitudinal nature, the bulk plasmons cannot be excited by visible light. The surface-propagating plasmons propagate along metal surfaces in a waveguide-like fashion [ 98 ]. In the case of NPs, when they are irradiated by visible light, the oscillating electric field causes the conductive electrons to oscillate coherently. When the electron cloud is displaced relative to the nuclei, a restoring force rises from Coulomb attraction between electrons and nuclei that results in oscillation of the electron cloud relative to the nuclear framework [ 99 ]. This creates uncompensated charges at the NP surface (Fig. 5 D). As the main effect producing the restoring force is the polarization of the NP surface, these oscillations are called surface plasmons and have a well-defined resonance frequency [ 98 ].
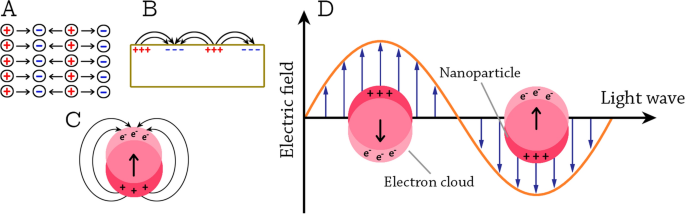
Graphical illustration of the types of plasmons. A bulk; B surface propagating; and C surface localized plasmons (adapted from Khlebtsov et al., 2010 [ 98 ]). D graphical illustration of the localized surface plasmon resonance (LSPR) in NPs (adapted from Kelly et al., 2003 [ 99 ])
Experimental studies on Ag NPs showed significant differences in their optical properties based on the size of NPs. For Ag NPs with 30 nm radius, the main extinction peak was at 369 nm wavelength, while for Ag NPs with 60 nm radius, a totally different behavior was observed [ 99 ]. The same researchers found that the shape of the NPs also is critical for the optical properties, the plasmon resonance wavelength shifts to the red as the NPs become more oblate [ 99 ], demonstrating that plasmon resonance strongly depend on NPs shape. With respect to the dielectric environment of the NPs, both the surrounding solvent and the support (substrate) were found to be critical for the optical properties. For Ag NPs, both experimental and theorical studies on the effect of surrounding solvent show that plasmon wavelength linearly depends on the refractive index of the solvent [ 99 , 101 ]. At the same time, 10 nm Ag NPs supported on mica substrates displayed LSPR wavelength shifts to the red compared to unsupported NPs [ 102 ]. The biogenic synthesis of NPs can also improve the optical properties. Biologically produced CeO 2 NPs using Simarouba glauca leave extract were found to have different absorption bands and higher band gap energies compared to chemically produced CeO 2 NPs. These superior optical properties were attributed to the better crystallinity and small size of biogenic NPs compared to chemical NPs [ 103 ]. Biogenic NPs can also offer higher photocatalytic activities, e.g., ZnO NPs produced by Plectranthus amboinicus leaf extract had higher photocatalytic activity in the photodegradation of methyl red under UV illumination compared to chemical produced ZnO NPs [ 104 ].
Catalytic properties
Nano-catalysis, i.e., the use of NPs as catalysts, is a quickly evolving field within chemical catalysis. Significantly enhanced or novel catalytic properties such as reactivity and selectivity have been reported for NP catalysts compared to their bulk analogues. The catalytic properties of NPs depend on the size, shape, composition, interparticle spacing, the oxidation state, and the support of the NPs [ 76 ].
The dependency of catalytic activity on the size of NPs is well studied. The relation is an inverse one, i.e., the smaller the NPs the more catalytically active they are. This relationship was found e.g., in the electro-catalysis oxidation of CO by size-selected Au NPs (1.5, 4, and 6 nm) deposited on indium tin oxide. The researchers observed that the smallest NPs provided the highest normalized current densities [ 105 ]. The same relationship was also found in several other studies [ 106 , 107 , 108 , 109 , 110 ]. Goodman et al., 1998 [ 111 ] speculated originally that this behavior could be attributed to quantum-size effects generated by the confinement of electrons within a small volume. Later, size-dependent changes in the electronic structure of the clusters [ 112 ] and the resulting larger number of low-coordinated atoms available for interaction by the larger surface-to-volume ratios with smaller NPs were discussed [ 76 ].
The shape is also known to affect the reactivity and selectivity of the NPs. For the oxidation of CO by Au NPs, hemispherical NPs were found to be more active than spherical ones [ 113 ]. For the oxidation of styrene by Ag NPs, nanocubes were found to be fourteen times more efficient than nanoplates and four times more efficient than nanospheres [ 114 ]. The reason for these dramatical changes are attributed to the increase/decrease in the relative area of the catalytically active surface facets [ 76 ] or to the differences in stability for different NP shapes [ 115 ].
As for composition, several studies have shown that the use of alloys in NPs can enhance the catalytic activity as a result of the alloying effect causing changes in the electronic properties of the catalyst, decreasing poisoning effects, and providing distinct selectivities [ 76 ]. For example, the alloying of Pt with other metals such as Ru, Ni, and Co, was reported to enhance the hydrogenation and oxygen reduction activity of the NP catalyst material, as well as enhancing the resistance against CO poisoning [ 116 , 117 , 118 ]. However, the alloying of Pt with Fe, Ru, and Pd, resulted in reduced reactivity for methanol decomposition [ 119 ]. This reduction in reactivity was explained by the possible occupation of the surface with the addition metal atoms, since pure Fe, Ru, and Pd clusters are less reactive for methanol decomposition than similarly-sized pure Pt clusters. In general, the change in the composition of NPs changes the electronic structure of metal surfaces by the formation of bimetallic bonds as well as the modification of metal–metal bond lengths [ 76 ]. In addition, the charge-transfer phenomenon between different metals may favorably change the binding energy of adsorbents, lower the barriers for specific chemical reactions, and enhance resistance against poisoning [ 120 , 121 , 122 ].
The catalytic activity and stability of 2 nm Au NPs dispersed on polycrystalline TiC films displayed a strong dependence on interparticle spacing. In this study, Au NPs having two different interparticle spacing (30 and 80 nm) were analyzed by Thermal Desorption Spectroscopy. It was found that the sample with smaller interparticle spacing was poisoned and subsequently deactivated while the sample with longer interparticle spacing showed longer lifetime [ 123 ]. At the same time, the oxidation state of NPs was shown to affect the catalytic activities. Ru NPs under rich O 2 conditions and moderate temperatures oxidize and form RuO 2 , the reaction of CO oxidation was found to occur on the metal oxide surface not the metal surface [ 124 ]. A similar effect on CO oxidation was also observed with Pt NPs in which the reactivity of PtO 2 was found to be higher than Pt [ 125 ]. The reaction of CO oxidation was compared for several metal NPs (Ru, Pd, Ir, Os, and Pt) and their corresponding oxides, and the oxides were indeed more reactive than the metals [ 126 , 127 ]. The superior catalytic performance of RuO 2 over their metallic counterparts is generally agreed on, nevertheless, the same cannot be said for other catalytically active metals such as Pt [ 76 ]. In general, these differences in catalytic performance are attributed to the electron transfer processes at the metal/metal oxide interfaces. Consequently, the view that NP oxidation is an undesirable process that leads to the reduction of catalytic performance needs to be reconsidered [ 128 ].
An example for the effect of the support material is the role of the MgO support for Au NPs, where MgO was found to be important for CO oxidation and particularly, for controlling the rate of CO oxidation through oxygen vacancies [ 129 ]. Later, the process of electron charge transfer from oxygen vacancies at the metal-substrate interface of supported Au NPs was suggested to be an ideal environment for O 2 activation and oxidation reactions [ 130 ]. A similar behavior was also found in the decomposition of SO 2 and dissociation of water by Au NPs supported on CeO 2 , in which CeO 2 supports played a critical role [ 131 ]. The experiments showed that not only the chemical composition of the support affects the reactivity of the catalyst, but the crystal structure of the support, too [ 132 ]. Enhanced catalytic performance for CO oxidation and SO 2 dissociation have also been reported for Au NPs supported on metal carbides such as TiC [ 108 , 133 ]. In addition to enhanced catalytic reactivities, the support also plays an important role in NP stabilization [ 106 ], i.e., the stabilization of NPs against coarsening, the stabilization of metal oxides at the NP surface, and the stabilization of intermediate reactions species [ 76 ].
Characterization of NPs
The properties of NPs determine their potential applications. Hence, different methods and techniques are used for the analysis and characterization of the various physicochemical properties of NPs. Table 1 summarizes all characterization techniques mentioned in this review and shows what properties and features can be resolved by each technique.
Morphological and topographical characterization
The morphological and topographical features of NPs are of great interest since they influence most of the properties of NPs as described above. These features include the size, shape, dispersity, localization, agglomeration/aggregation, surface morphology, surface area, and porosity of the NPs. The following techniques are regularly used for the characterization of morphological and topographical features of NPs.
Electron microscopy (EM)
Scanning electron microscopy (SEM), scanning tunneling microscopy (STM), and transmission electron microscopy (TEM) are frequently employed for the analysis of NP size, shape, and surface. In SEM, an electron gun is used to produce a beam of electrons that is controlled by a set of lenses to follows a vertical path through the microscope until it hits the samples. Once the sample is hit by the beam, electrons and X-rays are ejected from the sample. Detectors are then used to collect the X-rays and scattered electrons in order to create a 3D image of the sample. SEM provides different information about the NPs such as size, shape, aggregation, and dispersion [ 134 ]. Similarly, TEM provides information about the size, shape, localization, dispersity, and aggregation of NPs in two-dimensional images [ 25 ]. TEM employs an electromagnetic lens that focuses a very fine beam of electrons into an ultrathin section of the sample. This beam passes through the specimen where the electrons either scatter or penetrate the sample and hit a fluorescent screen at the bottom of the microscope. The difference in electron densities is used for the contrast to create an image of the specimen. TEM can be also used for the characterization of NP crystal structure through the use of selected area electron diffraction (SAED), where the electron beam is focused on a selected area in the sample and the scattered electrons are used to obtain a diffraction pattern. STM is based on the phenomenon of quantum tunneling, where a metallic tip is brough very close to the sample surface and used to apply voltage. When voltage is applied, electrons from the sample surface are extracted creating an electrical current that is used to reconstruct an image of the surface with atomic resolution [ 135 ]. STM is mainly used to characterize the topography of NPs. For inorganic NPs, these techniques offer excellent approaches for the determination of morphological features of NPs. For organic NPs (or NPs coated with biological materials), these techniques require sophisticated sample preparations which constitute major restrictions to their use [ 136 ]. The sample preparation for these techniques might cause sample dehydration, which might lead e.g. to sample shrinking and aggregation [ 136 ].
Examples: TEM was used for the characterization of Ag NPs produced by Arbutus unedo leaf extract. In this example, the NPs have a spherical morphology with a uniform size of 30 nm. The NPs were found to agglomerate into small aggregates, each including 5–6 NPs. At the same time, the SAED approach was used to determine the crystal structure of the NPs. The majority of the NPs were found to be single crystalline cubic materials predominately oriented along their (111) direction [ 137 ]. For the characterization of Ag NPs produced by Diospyros kaki leaf extract, SEM helped to show that the NPs were also spherical and the size was 32 nm with some deviations [ 138 ]. STM is less frequently used for the characterization of biogenic NPs. The features of Ag NPs produced by lime, sweet-lime, and orange juices were compared using STM technique [ 139 ].
Dynamic light scattering (DLS)
This technique is a common approach for the analysis of NP size and size distribution. This approach involves the measurement of light interference based on the Brownian motion of NPs in suspension, and on the correlation of NP velocity (diffusion coefficient) with their size using Strokes-Einstein equation [ 140 ]. The size distribution range of NPs is shown as the polydispersity index, which is the output of an autocorrelation function [ 136 ]. The polydispersity index values lie between 0 and 1, where 0 represents a completely homogenous population and 1 represents a highly heterogeneous population. This technique also allows the analysis of non-spherical NPs through the use of multistage DLS [ 136 ]. This technique is also referred to as photon correlation spectroscopy (PCS) [ 141 ].
Examples: DLS was used to measure the size and the size distribution profile of a wide range of biogenic NPs. The average size of Ag NPs produced by Trichoderma koningii fungi was found to be around 25 nm and the size distribution profile was between 14 and 34 nm. The polydispersity index for those NPs was 0.681, which indicates that they are polydispersed [ 142 ]. While the average size of Ag NPs produced by potato ( Solanum tuberosum ) was found to be around 10–12 nm with a wider distribution profile between 3–65 nm [ 143 ]. In a different application, DLS was employed to study the size increase of biogenic MnO 2 NPs overtime, demonstrating that their size is 7.5 nm after 3 min of the initiation of the reaction, then their size grows overtime until it become 54 nm after 31 min [ 144 ].
Nanoparticle tracking analysis (NTA)
This method is used for the analysis of NP size in suspensions based on their Brownian motion. Like in DLS, the rate of NP movement is correlated with their size using Strokes-Einstein equation, allowing the measurement of size distribution profiles for NPs with 10–1000 nm diameter. Its advantage over DLS is that NP motion is analyzed by video. Individual positional changes of NPs are tracked in two dimensions, which are used to determine NP diffusion rates, and by knowing the diffusion coefficient, the hydrodynamic diameter of the particles can be calculated. In DLS, individual NPs are not visualized, but instead, the time-dependent intensity fluctuations caused by Brownian motion are used to calculate the polydispersity index [ 145 ]. NTA was found to be more precise for sizing monodisperse as well as polydisperse organic NPs compared to DLS [ 146 ].
Examples: NTA was used to measure the size and dispersity of Ag NPs produced by Camellia sinensis (green tea) powder, the NPs were found to be well dispersed in an aqueous medium with an average size of 45 ± 12 nm [ 147 ]. For Se NPs produced by lactic acid bacteria, NTA was employed to measure the size and the concentration of NPs. The average size was found to be 187 ± 56 nm with a concentration of (4.67 ± 0.30) × 10 9 Se NPs per ml [ 148 ].
Brunauer–Emmett–Teller (BET) method
This method is based on the adsorption and desorption principle developed by Stephen Brunauer, Paul Emmett, and Edward Teller, and it is considered one of the best methods for the analysis of NP surface area [ 25 ]. In BET analysis, a partial vacuum is created to produce adsorption between the sample and liquid N 2 (because the interaction between solid and gaseous phases is weak, the surface is cooled with liquid N 2 to obtain detectable amounts of adsorption). After the formation of adsorption monolayers, the sample is removed from the N 2 atmosphere and heated to cause the adsorbed N 2 to be released from the material (desorption) and quantified. The data collected is displayed in the form of isotherms (graphs representing the amount of N 2 adsorbed as a function of relative pressure at a constant temperature). The data is displayed in five isotherms where the information is used to determine the surface area of the sample [ 25 , 149 ]. Figure 6 graphically illustrates the principle of this method.

Principles of the BET and BJH methods. The BET method (steps 1–3) is based on the adsorption of nitrogen on the NP surface. After the formation of a monolayer, nitrogen is desorbed, and the surface area is calculated. The BJH method (steps 1, 2, 4, and 5) is based on the complete filling of NP pores with liquid nitrogen. When saturation is reached, nitrogen is desorbed, and pore size is calculated
Examples: The BET method was employed to measure the surface area of CeO 2 NPs produced by Eucalyptus globulus leaf extract. The surface area was found to be 40.96 m 2 /g of biogenic CeO 2 NPs, much higher than the commercial CeO 2 NPs (8.5 m 2 /g) [ 150 ]. BET was also used to measure the surface area of SiO 2 NPs produced by rice husk, CuO NPs produced by Leucaena leucocephala leaf extract, and Ag NPs produced by Acanthospermum hispidum leaf extract. In these examples, the surface area was 7.15 m 2 /g, 47.54 m 2 /g, and 9.91 m 2 /g, respectively [ 151 , 152 , 153 ].
Barrett–Joyner–Halenda (BJH) method
This method is based on the Barrett–Joyner–Halenda principle and is used for the determination of porosity (or pore size) of NPs. Similar to the BET method, this method also involves the use of N 2 gas to adsorb to the sample. In the BJH method, the process is extended so the gas condensates in the sample pores as pressure increases. The pressure is increased until a saturation point is achieved, at which all the pores of the sample are filled with liquid. Afterwards, the condensated gas is allowed to evaporate where the desorption data is calculated and correlated to the pore size using a modified Kelvin equation (Kelvin model of pore filling) [ 154 , 155 ]. Figure 6 graphically illustrates this method.
Examples: The BJH method was employed to study the pore size of a wide range of biogenic NPs, for instance, the pore size of CeO 2 NPs produced by Eucalyptus globulus leaf extract was found to be 7.8 nm [ 150 ], the pore size of CuO NPs produced by Leucaena leucocephala leaf extract was 2.13 nm [ 152 ], the pore size of SiO 2 NPs produced by rice husk and Ag NPs produced by Acanthospermum hispidum leaf extract were much larger, being 29.63 nm and 36.34 nm, respectively [ 151 , 153 ].
Structural and chemical characterization
The structural characterization of NPs and the study of their composition is of high interest due to the strong influence of these parameters on the physicochemical properties. The following techniques are commonly used for the analysis of NP composition, phase, crystallinity, functionalization, chemical state (oxidation), surface charge, polarity, bonding, and electrochemical properties.
X-ray diffraction analysis (XRD)
This technique is based on irradiating a material with incident X-rays and then measuring the intensities and scattering angles of the X-rays that leave the material [ 156 ]. This technique is widely used for the analysis of NP phase and crystallinity. However, the resolution and accuracy of XRD can be affected in cases where the samples have highly amorphous characteristics with varied interatomic distances or when the NPs are smaller than several hundreds of atoms [ 25 ].
Examples: For the characterization of biogenic Ag NPs, the XRD results of Ag NPs produced by Trichoderma koningii [ 142 ], Solanum tuberosum [ 143 ], and Acanthospermum hispidum leaf extract [ 153 ] displayed characteristic peaks occurring at roughly 2θ = 38 o , 44°, and 64 o corresponding to (111), (200), and (220) planes, respectively. These results are in good agreement with the reference to the face-centered cubic structure of crystalline silver. However, the XRD results of Ag NPs produced by Solanum tuberosum were not as clear as the other biogenic Ag NPs and had several impurities. The structural characterization of Pd NPs produced by Garcinia pedunculata Roxb leaf extract by XRD showed the distinct peaks of Pd, however, three other peaks were also observed at 2θ of 34.22˚, 55.72˚, and 86.38˚, indicating the presence of PdO phases along with Pd NPs [ 157 ].
Energy-dispersive X-ray spectroscopy (EDX)
This technique is based on the irradiation of the sample with an electron beam. Electrons of the electron beam when incident on the sample surface eject inner shell electrons, the transition of outer shell electrons to fill up the vacancy in the inner shell produces X-rays. Each element produces a characteristic X-ray emission pattern due to its unique atomic structure, and therefore can be used to perform compositional analysis [ 158 ]. The shortfall of EDX is that the resulting spectra give only qualitative compositional information (it shows the chemical elements present in the sample without quantification). However, the peak intensities to some extent give an estimate of the relative abundance of an element in a sample [ 159 ]. This technique does not require sophisticated additional infrastructures, usually it is a small device that is connected to an existing SEM or TEM. This allows the use of SEM or TEM for the morphological characterization and EDX is used simultaneously for the analysis of chemical composition [ 160 ].
Examples: The EDX technique is usually used for the confirmation of the presence of the element in question in biogenic NPs. For instance, EDX was used to confirm the presence of Au in Au NPs produced by Jasminum auriculatum leaf extract [ 161 ], the presence of Pd in Pd NPs produced by Pulicaria glutinosa extract [ 162 ], the presence of Te in Te NPs produced by Penicillium chrysogenum PTCC 5031 [ 163 ], and the presence of Ag in Ag NPs produced by Trichoderma viride [ 164 ].
High-angle annular dark-field imaging (HAADF)
This method is used for the elemental mapping of a sample using a scanning transmission electron microscope (STEM). The images are formed by the collection of incoherently scattering electrons with an annular dark-field detector [ 165 ]. This method offers high sensitivity to variations in the atomic number of elements of the sample, and it is used for elemental composition analysis usually when the NPs of interest consist of relatively heavy elements. The contrast of the images is strongly correlated with atomic number and specimen thickness [ 166 ].
Examples: The employment of HAADF-STEM in the characterization of biogenic Au–Ag–Cu alloy NPs confirmed the presence of the three elements in the same NP [ 167 ]. Similarly, this approach revealed that Ag NPs produced by Andrographis paniculata stem extract were coated with an organic polymer [ 168 ]. The employment of this approach in the characterization of Cu NPs produced by Shewanella oneidensis revealed that Cu NPs remained stable against oxidization under anaerobic conditions, but when they were exposed to air a thin shell of Cu 2 O develop around the NPs [ 169 ].
X-ray photoelectron spectroscopy (XPS)
This technique is considered the most sensitive approach for the determination of NP exact elemental ratios, chemical state, and exact bonding nature of NP materials [ 25 ]. XPS is based on the photoelectric effect that can identify the elements within a material, or covering a material, as well as their chemical state with high precision [ 170 ]. XPS can also be used to provide in-depth information on electron transfer, e.g., for Pt NPs supported on CeO 2 , it was found that per ten Pt atoms only one electron is transferred to the support [ 171 ].
Examples: The XPS technique can employed for different purposes. For instance, it was used for measuring the purity of Au NPs produced by cumin seed powder [ 172 ]. XPS was used for the determination of the oxidation states of Pt NPs produced by Nigella sativa seeds and Ag NPs produced by Rosa canina . XPS results of Pt NPs showed the presence of three oxidation states for Pt (Pt (0), Pt (II), and Pt (IV)) and two oxidation states for Ag NPs (Ag (0) and Ag (I)). In both cases, the zero-oxidation state was the abundant one, the presence of a small amount of the other oxidation states suggests that some of the NPs were oxidized or had unreduced species [ 173 , 174 ]. XPS was used for the determination of the exact elemental ratios and the bonding nature of FeS NPs produced by Shewanella putrefaciens CN32. For the exact elemental ratios, the researchers compared biogenic and abiotic FeS NPs and found that biogenic FeS NPs had a 2.3:1 Fe:S ratio while the abiotic NPs had a 1.3:1 Fe:S ratio. For the bonding nature, it was determined that the surface of NPs had Fe(II)-S, Fe(III)-S, Fe(II)-O, and Fe(III)-O bonds [ 175 ].
Fourier-transform infrared spectroscopy (FTIR)
This technique is based on irradiating a material with infrared light, where the absorbed or transmitted radiation is recorded. The resulting spectrum represents a unique fingerprint of samples, where information about the nature of the sample can be obtained such as the bonds involved, polarity, and oxidation state of the sample [ 176 , 177 ]. This technique is mainly used for the characterization of organic materials such as the surface chemical composition or functionalization of NPs. It is also used for the identification of contaminants when high purity is sought [ 178 ].
Examples: For biogenic NPs, FTIR is usually used for the identification of probable functional groups present on the surface of NPs that are responsible for the reduction and stabilization of the NPs. For plant-mediated NP synthesis, for instance for Ag NPs produced by Camellia sinensis , the FTIR results indicate the presence of Camellia sinensis phytocompounds, such as caffeine and catechin, on the surface of Ag NPs that could be responsible for the reduction of Ag or act as stabilizing agents [ 147 ]. For Ag NPs produced by Solanum tuberosum , the NPs were found to be capped by amide and amine groups [ 143 ]. For CeO 2 NPs produced by Eucalyptus globulus , the polyphenol groups present in Eucalyptus globulus extract were found on the surface of NPs suggesting their involvement in the reduction/stabilization process [ 150 ]. For microbe-mediated NP synthesis, FTIR results show the presence of protein residues on the surface of NPs confirming the involvement of different proteins in the reduction/stabilization process, such as in Ag NPs produced by Streptomyces sp. NH28 [ 179 ], in Te NPs produced by Penicillium chrysogenum PTCC 5031 [ 163 ], and in Se NPs produced by Azospirillum thiophilum [ 180 ].
Zeta potential analysis
Zeta potential measurements are used for the determination of NP surface charge in colloidal solutions. The surface charge of NPs attracts counter-ions that form a thin layer on the surface of the NPs (called Stern layer). This layer travels with the NPs as they diffuse thought the solution. The electric potential at the boundary of this layer is known as NP zeta potential [ 136 ]. The instruments used to measure this potential are called zeta potential analyzers [ 181 ]. Zeta potential values are indicative for NP stability, where higher absolute value of zeta potential indicate more stable NPs [ 136 ].
Examples: The zeta potential is a good indicator for the stability of NPs, where NPs with zeta potentials of more than + 30 mV or less than − 30 mV are considered stable. Zeta potentials have been measured for a wide range of biogenic NPs. The zeta potential for Ag NPs produced by Ziziphus jujuba leaf extract of − 26.4 mV [ 182 ]. Ag NPs produced by other organisms have different zeta potential values, for example, Ag NPs produced by Punica granatum peel extract have a zeta potential of − 40.6 mV indicating their higher stability [ 183 ], while Ag NPs produced by Aspergillus tubingensis have a zeta potential of + 8.48 indicating their relative instability [ 184 ]. The pH of the sample is another important parameter for zeta potential values, the higher pH the lower the zeta potential value [ 185 ]. Having different zeta potential values for the same type of NPs depending on the organism used for their synthesis is not unique to silver, Se NPs also show different potential values depending on the organism used for their synthesis [ 186 ].
Cyclic voltammetry (CV)
CV is an electrochemical technique for measuring the current response of redox-active solutions to a linearly cycled potential sweep between two or more set values. The CV technique involves the use of three electrodes: a working electrode, reference electrode, and counter electrode. These electrodes are introduced to an electrochemical cell filled with an electrolyte solution and where voltage is in excess, the potential of the working electrode is cycled and the resulting current is measured. This technique is used for determining information about the reduction potential of materials, the kinetics of electron transfer reactions, and the thermodynamics of redox processes [ 187 , 188 , 189 ].
Examples: The CV technique can be employed for two different purposes in the context of biogenic NP characterization. Firstly, it can be used for measuring the stability of NPs in electrocatalysis. For this purpose, the biogenic NPs are assembled on an electrode of the electrolysis cell and are tested for their electrocatalytic behavior against a redox reaction over different cycles. As an example, Ag NPs produced by Citrus sinensis were found to be stable in phenolic compounds redox reactions over multiple cycles [ 190 ]. Secondly, CV can be used for monitoring the progress of reduction of metallic NPs or for the determination of the reducing agent involved in the reduction. For example, for Ag NPs produced by Indian propolis, four cyclic voltammograms were recorded, one for a water extract of Indian propolis, another for an ethanol extract of Indian propolis, and two for the constituent flavonoids of Indian propolis (pinocembrin and galangin). The four cyclic voltammograms showed similar behaviors indicating the involvement of these flavonoids in the reduction of Ag and in forming Ag NPs [ 191 ].
Raman spectroscopy
This technique is based on irradiating a sample with monochromatic light emitted by a laser, in which the interactions between the laser light and molecular vibrations (photons and phonons) are recorded. The technique records the inelastically scattered photons, known as Raman scattering (named after the Indian physician C. V. Raman) [ 192 ]. The output of this technique is a unique fingerprint for each sample, which is used to characterize the chemical and intramolecular bonding of the sample. It can also be used to characterize the crystallographic orientation of the sample [ 193 ]. Surface-enhanced Raman spectroscopy (SERS) enhances Raman scattering of a sample and provides a more sensitive, specific, and selective technique for identifying molecular structures [ 194 ]. Both techniques are also used for the characterization of optical properties, where the recorded photons and phonons are used to understand the plasmonic resonance of NPs [ 25 ].
Examples: Raman spectroscopy was used to characterize Fe 3 O 4 NPs produced by Pisum sativum peel, the researchers found that the NPs were Fe 3 O 4 NPs with face centered cubic phase which was in agreement with their XRD measurements [ 195 ]. Other researchers used Raman spectroscopy for studying the trace deposits of carbohydrates on ferrihydrite NPs produced by Klebsiella oxytoca , the results showed that the pores of NPs had more deposits of carbohydrates that the surface of the NPs [ 196 ]. For Au NPs produced by Raphidocelis subcapitata (green algae), several biomolecules were suggested for their involvement in this process. SERS technique was used to study Au NPs surface-associated biomolecules in order to narrow down the list of biomolecules involved in the bioproduction process. The researchers found that several biomolecules such as, glutathione, β-carotene, chlorophyll a, hydroxyquinoline, and NAD were associated with Au NPs surface, thus, ruling out other molecules such as, glutaraldehyde fixing agent, saccharides, FAD, lipids, and DNA from the list [ 197 ].
Characterization of optical, electronic, and electrical properties
In addition to Raman spectroscopy and SERS, also other techniques can be employed to study and characterize the optical properties of NPs. These techniques give information about the absorption, reflectance, fluorescence, luminescence, electronic state, bandgap, photoactivity, and electrical conductance properties of NPs.
Ultraviolet–visible spectroscopy (UV–vis) and photoluminescence spectroscopy (PL)
In absorption spectroscopy such as UV–vis, the transition of electrons from the ground state to an excited state is measured, while in photoluminescence spectroscopy, the transition of electrons from the excited state to the ground state is measured [ 198 ]. UV–vis spectroscopy uses visible and UV light to measure the absorption or reflectance of a sample. In photoluminescence spectroscopy, usually UV light is used to excite the electron and then measure the luminescence or fluorescence properties of a sample [ 199 ].
Examples: UV–vis spectroscopy is a simple and common technique that is used for the characterization of the optical properties of NPs. For instance, for the characterization of the optical properties of Ag NPs produced by Trichoderma viride , the UV–vis spectrum showed that a Ag surface plasmon band occurs at 405 nm, which is a characteristic band for Ag NPs. The intensity of this band over the reaction time increased as a result of increasing Ag NP concentration in the solution. In the same study, the photoluminescence properties of these NPs were recorded, with an emission in the range between 320–520 nm, which falls in the blue-orange region [ 164 ]. For biogenic Cu NPs, the common absorption peaks are located between 530–590 nm. The difference in NP size and the bio-active molecules used for the reduction process are believed to be the reasons behind the differences in the absorption peaks [ 200 ]. For instance, 15 nm spherical Cu NPs produced by Calotropis procera have an absorption peak at 570 nm [ 201 ], while 76 nm spherical Cu NPs produced by Duranta erecta have an absorption peak at 588 nm [ 202 ]. The same applies to photoluminescence effects, where 27 nm spherical Cu NPs produced by Tilia extract emit light of 563 nm (dark brown) [ 203 ], while 19 nm spherical Cu NPs emit light of 430 nm (green) [ 204 ].
UV–vis diffuse reflectance spectroscopy (DRS)
This technique uses UV and visible light to measure the diffuse reflectance of a material (the reflection of light in many angles, as opposed to specular reflection). The resulting diffuse reflectance spectra are used to determine the electronic state of a sample, which is then used to calculate the bandgap [ 25 ]. Bandgap determination is crucial for determining conductance and photocatalytic properties especially for semiconductor NPs [ 205 ].
Examples: The DRS technique was used to calculate the bandgap for a wide range of biogenic NPs. For instance, TiO 2 NPs produced by Andrographis paniculata exhibit an optical energy bandgap of 3.27 eV [ 206 ]. Interestingly, biogenic ZnO NPs produced by different organism show different bandgaps, for example, ZnO NPs produced by Pseudomonas putida have a bandgap of 4 eV [ 207 ], while ZnO NPs produced by Calotropis procera leaf extract have a bandgap of 3.1 eV [ 208 ].
Spectroscopic ellipsometry
This technique is based on irradiating a sample with polarized light to measures changes in polarization. It is widely used to calculate the optical constants of a material (refractive index and extinction coefficient) [ 209 ]. This technique is also used to characterize the electrical conductivity and dielectric properties of materials [ 210 ].
Examples: Spectroscopic ellipsometry is not a common technique for the characterization of biogenic NPs. For chemically produced NPs, the optical properties for different-sized Au NPs partially embedded in glass substrate were measured by spectroscopic ellipsometry. In this example, a clear transition from LSPR to SPR mode was found as the thickness increases. Moreover, the partially-embedded Au NPs had much higher refractive index sensitivity compared to Au NPs fully immobilized in a glass substrate [ 211 ]. Spectroscopic ellipsometry was also used to measure the changes in the optical constants of a layer of 5 nm ZnO NPs induced by UV illumination. In this case, it was found that the UV illumination of ZnO NPs in inert atmospheres resulted in a clear blue shift in the absorption (Moss-Burstein shift). The UV illumination of ZnO NPs results in the desorption of O 2 from the NPs surface leading to the population of the lowest levels in conduction band with mobile electrons. This phenomenon is reversible, in which the exposure to O 2 from air results in the scavenging of these mobile electrons [ 212 ].
Characterization of magnetic properties
The magnetic properties of NPs are of high importance, as they potentially give NPs great advantages in catalysis, electronics, and medical applications. Several techniques were developed for the detection and quantification of small magnetic moments in NPs.
Magnetic force microscopy (MFM)
This technique is a variety of atomic force microscopy (AFM), in which a magnetic tip is used to scan the sample. The magnetic tip is approached very close to the sample, where the magnetic interactions between the tip and the sample are recorded [ 213 ]. At closer distances to the sample (0–20 nm), other forces such as van der Waals forces also interact with the tip. Therefore, MFM measurements are often operated with two-pass scanning method (also called lift height method) [ 214 ] (Fig. 7 ). In this method, the tip is firstly used to measure the topography of the sample including the molecular forces as van der Waals. Afterwards, the tip is lifted and a second scan is operated following the same topography outline. In the second scan, the short-ranged van der Waals forces disappear and the long-range magnetic forces are almost exclusively recorded. In an experimental study, researchers found that 22 nm was the optimal scanning height for the second scan, at which van der Waals forces are very weak while the distance is still small enough to measure the magnetic interactions for Pd-Fe bimetallic NPs [ 215 ].
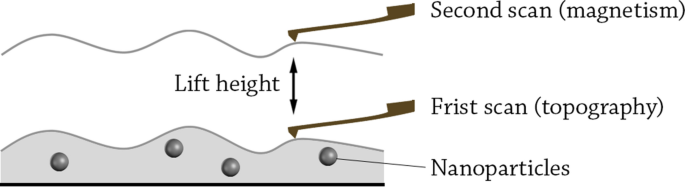
Magnetic force microscopy lift height method. The first scan is done very close to the surface to obtain the topography of the sample. Then, the tip is lifted and a second scan is performed following the topography outline obtained in the first scan
Examples: MFM was heavily used for the characterization of magnetite NPs produced by magnetotactic bacteria. For instance, the size and orientation of the magnetic moment of magnetite NPs produced by Magnetospirillum gryphiswaldense strain MSR-1 were studied by MFM [ 216 ], in which the size of the magnetic moment was found to be 1.61 × 10 −17 Am 2 . In a different study, MFM was used to characterize the magnetic properties and to estimate the size of the magnetic kernel of the magnetosomes produced by the same strain, and it was determined that the NPs behaved like single mono-domain nanomagnets [ 217 ]. The magnetic properties of NPs made from materials such as Pd that only exhibit significant magnetism on the nanoscale can also be studied by MFM, however, the magnetic moment of these NPs is much lower than for ferromagnetic NPs. The magnetic decoration of Pd NP samples with Fe 2 O 3 NPs strongly enhances the weak magnetic signal of Pd NPs up to 15 times [ 218 ]. This approach could make the MFM technique useful for the characterization of weak magnetic NPs.
Vibrating-sample magnetometry (VSM)
This technique measures the magnetic properties of materials based on Faraday’s law of induction. In VSM, the sample is placed in a constant magnetic field in a special holder that vibrates vertically. As the holder starts vibrating, the magnetic moment of the sample creates a magnetic field that changes as function of time. The alternating magnetic field created in the sample induces an electric current that is recorded and used to calculate the magnetic properties of the sample [ 219 , 220 ].
Examples: For the characterization of Fe 2 O 3 NPs produced by Tridax leaf extract, VSM studies revealed that the NPs had a saturation magnetization of 7.78 emu/g, a remnant magnetization of 0.054 emu/g, and a coercivity of − 1.6 G [ 221 ]. In other studies, VSM was used to compare the magnetic properties of iron oxide NPs produced Moringa oleifera with the magnetic properties of the same NPs but coated with chitosan. The researchers found that saturation magnetisation, remnant magnetization, and coercivity have lower values when the NPs are coated with chitosan [ 222 ].
Superconducting quantum interference device (SQUID) magnetometry
This technique measures the magnetic properties of materials based on the Josephson effect. Niobium (Nb) or other metal alloys are used in the device which needs to be operated at temperatures very close to the absolute zero to main superconductivity, where liquid helium is used to maintain the cold environment [ 223 ]. However, other kinds of SQUID also exist where high-temperature superconductors are used [ 224 ]. After reaching superconducting environments, the Josephson junctions contained in the device help to create a supercurrent, which is recorded and used to calculate the magnetic properties of the sample [ 225 ].
Examples: For the characterization of iron oxide NPs produced by Cnidium monnieri seed extract, SQUID magnetometry revealed that the NPs had a saturation magnetization of 54.60 emu/g, a remnant magnetization of 1.15 emu/g, a coercivity of 11 Oe, and a magnetic susceptibility of + 1.69 × 10 –3 emu/ cm 3 ⋅ Oe at room temperatures, indicating the superparamagnetic behaviour of these NPs [ 226 ]. SQUID magnetometry was also used for the characterization of the magnetic properties of zinc incorporated magnetite NPs produced by Geobacter sulfurreducens , showing that the loading of only 5% zinc results in the enhancement of saturation magnetization of the NPs by more than 50% [ 227 ].
Electron spin resonance spectroscopy (ESR)
This technique measures the magnetic properties of materials by characterizing and quantifying the unpaired electrons in the sample. Electrons are charged particles that spin around their axis, which can align in two different orientations (+ ½ and − ½) when the sample is placed in strong magnetic field. These two alignments have different energies due to the Zeeman effect. Since unpaired electrons can change their spins by absorbing or emitting photons, in ESR the sample is irradiated with microwave pulses to excite electron spins until a resonance state is reached [ 228 ]. This technique is also referred to as electron paramagnetic resonance spectroscopy (EPR). It can be used to measure the ferromagnetic and antiferromagnetic properties of NPs [ 229 , 230 ].
Examples: ESR was used to characterize the magnetic properties of iron oxide NPs produced by Ficus carica . The trees naturally produce iron oxide NPs as a defence mechanism when are they are subjected to stress. The researchers found that the magnetic properties of iron oxide NPs produced by the same tree but grown in different environmental conditions have different magnetic properties. In addition, a magnetic anisotropy of the signal was visible as the magnetic properties of these NPs varied strongly at different temperatures [ 231 ]. ESR was also used to characterize the magnetic properties of Se nanomaterials produced by anaerobic granular sludge. The ESR results revealed the presence of Fe(III) atoms incorporated in the Se nanomaterial, which enhanced their overall magnetic properties, giving it ferromagnetic behaviour [ 232 ].
Characterization of thermal properties
Several techniques can be used for the characterization of the thermal properties of NPs, such as melting points, crystallization and structural-phase transition points, heat capacity, thermal conductivity, and thermal and oxidative stability.
Differential scanning calorimetry (DSC)
In this technique the analyte and a well-defined reference sample are put at the same temperature, then, the amount of heat required to increase the temperature of the sample and the reference in measured as a function of temperature. This technique is widely used to measure melting points [ 233 ], crystallization points, structural-phase transition points [ 234 ], latent heat capacity [ 235 ], heat of fusion [ 236 ], and oxidative stability [ 237 ].
Examples: For the characterization of Ag NPs produced by Rhodomyrtus tomentosa leaf extract, DSC showed three exothermic peaks at 44, 159, 243, and an endothermic peak at 441 °C. The first peak (at 44 °C) indicates that at this temperature the NPs face a gradual loss of water from their surface. The second peak (at 159 °C) shows that the thermal decomposition of the sample happens at this temperature. The last temperature (441 °C) indicates the melting temperature for those NPs [ 238 ]. For Ag NPs produced by Parthenium hysterophorus leaf extract, DSC showed that their melting temperature was at 750 °C. The researchers also found that these NPs had completely thermally decomposed and crystallized simultaneously [ 239 ].
Differential thermal analysis (DTA)
This technique is based on heating or cooling a sample and an inert reference under identical conditions, where any temperature difference between the sample and the reference is recorded. This technique is primarily used for the study of phase diagrams and transition temperatures [ 240 ]. However, it is also used to measure the melting points, thermal, and oxidative stability [ 241 , 242 ].
Thermogravimetric analysis (TGA)
This technique measures the change in the mass of a sample as a function of temperature and/or time in a controlled atmosphere [ 243 ]. This technique is mainly used to study the thermal stability of materials [ 244 ], in addition, it is also used to measure structural-phase transition points [ 245 ], thermal activation energies [ 246 ], and oxidative stability [ 247 ]. The resulting thermogram is unique for each compound and therefore can also be used for the determination of material composition [ 248 ]. TGA and DTA are usually combined in the same thermal analyzing instrument, called thermogravimetry/differential thermal analysis (TG/DTA) [ 244 ].
Examples: TG/DTA is a common technique for the characterization of thermal properties of biogenic NPs. For instance, the thermal properties of Ag NPs produced by Daphne mucronate leaf extract were studied in the range between 0–1000 °C where the sample was heated at a rate of 10 °C/min. The researchers found that between 400–500 °C the NPs faced a dominant weight loss, while the weight loss below 400 °C and above 500 °C was negligible. The DTA curve showed an intense exothermic peak in the range between 400–500 °C, this indicates that the crystallization of NPs happens in this temperature interval. Some minor weight loss events were seen below 400 °C, this may be caused by the evaporation of water or the degradation of the organic components [ 249 ]. In another study, the thermal properties of Ag NPs produced by two different plants ( Stereospermum binhchauensis and Jasminum subtriplinerve ) were compared. The researchers found that the major weight loss happens between 220–430 °C, which is attributed to the decomposition of biomolecules from the NP surface [ 250 ]. This shows that Ag NPs produced by these plants have much higher content of biomolecules on their surface than Ag NPs produced by Daphne mucronate. TG/DTA showed that Stereospermum binhchauensis Ag NPs crystallize at 315 °C and Jasminum subtriplinerve Ag NPs at 345 °C, around 100 °C less than Daphne mucronate Ag NPs [ 250 ].
Transient hot wire method (THW)
This method is used for the determination of thermal conductivity based on increasing the temperature of a material by a thin hot wire as a function of time, where the heating wire is located directly in the test sample. The advantage of this method over other thermal conductivity measurement methods is the very short measuring time, this gives high accuracy of thermal conductivity due to the negligible values of convection in such short times [ 251 ]. In this method, the NPs are added to a solution (usually water or ethylene glycol) forming a colloidal dispersion called a nanofluid. Then, the thermal conductivity of the nanofluid is measured and compared to the thermal conductivity of the base fluid, giving a thermal conductivity ratio which is used to evaluate the thermal conductivity of different NPs.
Examples: The thermal conductivity ratios of three different concentrations (0.12, 0.18, and 0.24%) of biogenic SnO 2 NPs produced by Punica granatum seed extract were measured in ethylene glycol at 303 K. The researchers found a linear relationship between NPs concentration and the thermal conductivity. The thermal conductivity enhancement of nanofluid to base fluid was between 6 and 24% [ 252 ]. In another study, the thermal conductivity of Fe 2 O 3 NPs produced by Psidium guajava leaf extract was measured in water and in ethylene glycol. The researchers found that the thermal conductivity enhancement in ethylene glycol was better than in water, the thermal conductivity enhancement for 0.025% Fe 2 O 3 NPs in water was 30% while in ethylene glycol was 34%. Moreover, the linear relationship between NPs concentration and thermal conductivity ratio was found for Fe 2 O 3 NPs in both water and ethylene glycol [ 253 ].
Characterization of mechanical properties
Several methods can be used for the characterization of mechanical properties of NPs, such as tensile and compressive strengths, elasticity, viscoelasticity, hardness, and stiffness.
Tensometery
The machine used for this method is called a universal testing machine (UTM) or a tensometer. It is used to measure the elasticity (elastic modulus), tensile and compressive strengths (Young’s modulus) of materials. In this machine, the sample is placed between grips and an extensometer, where changes in gauge length are recorded as a function of load [ 254 ]. However, other mechanical changes in addition to the change in gauge length are also recorded in this machine, such as the elasticity.
Examples: The mechanical properties of different biogenic NP-containing composites can be measured by this machine. For example, the mechanical properties of orthodontic elastic ligatures containing Ag NPs produced by Heterotheca inuloides were studied by comparing the maximum strength, tension, and displacement of the composite with and without the biogenic NPs. The researchers found that maximum strength, tension, and displacement have improved after the addition of Ag NPs [ 255 ]. Interestingly, the addition of biogenic Ag NPs produced by Diospyros lotus fruit extract to starch and polyvinyl alcohol hydrogel membranes resulted in an adverse effect. The tensile strength and modulus of the hydrogel membranes containing 50 and 100 ppm Ag NPs were much lower than of the neat hydrogel membrane. The researchers attributed this adverse effect to the possibility that the addition of Ag NPs could have resulted in blocking the crosslinking between starch and polyvinyl alcohol, or to the possibility of the formation of breakage points in the polymer matrix due to NPs agglomeration [ 256 ].
Instrumented indentation testing
This method is used to characterize the hardness features of materials by using a well-defined hard indenter tip typically made of diamond. The indenter tip is used to make an indentation in the sample by placing incremental loads on the tip, after which the area of indentation in the sample is measured and used to calculate the hardness features [ 257 ]. Light microscopy, SEM, or ATM technique are usually used to visualize the indentation in the sample. The method is also called micro- or nano-indentation testing.
Examples: This method was used to characterize the mechanical properties of calcite NPs produced by Ophiocoma wendtii brittlestar. The arm plates of this brittlestar are covered by hundreds of nanoscale calcite lenses that focus light onto photoreceptor nerve bundles positioned beneath the brittlestar. The researchers used the nanoindentation method to compare Young’s modulus, hardness and fracture toughness of biogenic calcite with geocalcite. The results showed that the biogenic calcite lenses have higher hardness and fracture toughness compared to geocalcite (more than twofold) [ 258 ]. Bamboo is well known for its high silica content in comparison to other wood species. It produces SiO 2 NPs and deposits it in its epidermis in the form of silica cells. The mechanical properties of silica cells compared to other types of cells of Moso bamboo ( Phyllostachys pubescens ) were studied by instrumented indentation testing. The researchers found that the cell wall of silica cells display higher hardness and elastic recovery compared to fibre and epidermal cells, which is attributed to the presence of biogenic SiO 2 NPs in the silica cells [ 259 ].
Dynamic mechanical analysis (DMA)
This method is used to study the mechanical properties of materials by measuring the strain of a material after applying a stress. This method helps to obtain three different values: storage modulus, loss modulus, and loss tangent. These values are important to give an overview about the stiffness and viscoelasticity behavior of materials [ 260 ].
Examples: The DMA method was used to characterize the mechanical properties of polymethyl methacrylate denture base polymer filled with Ag NPs produced by Boesenbergia rotunda . In this study frequency sweep test was used to determine the viscoelastic behavior of this nanocomposite where the temperature was constant at 37 °C and the frequency was increasing from 0.5 to 100 Hz in tension mode. The researchers found a frequency dependence for storage modulus, loss modulus, and loss tangent for the nanocomposite with various Ag NPs loading concentrations. The frequency dependence of storage modulus, loss modulus, and loss tangent indicates the viscoelastic response of this polymer. However, the results showed that the storage modulus for the nanocomposite is much higher than the loss modulus over the range of frequencies, indicating the elastic dominance of the nanocomposite. Moreover, the researchers found that storage and loss moduli increase with increasing Ag NPs loading concentrations, which is due to the interaction between polymethyl methacrylate and Ag NPs [ 261 ].
In a different study, DMA was used to determine the thermomechanical properties of pol(S-co-BuA) polymer filled with cellulose nanocrystals produced by Posidonia oceanica . In this case, the behaviour of storge modulus and loss tangent were studied as a function of temperature for different cellulose nanocrystals loading concentrations. The results showed that the unloaded polymer behaves like an amorphous polymer, the storage modulus remains constant until the temperature reaches 25 °C then it starts to sharply decrease due to glass–rubber transition. A relaxation process was also evident for the unloader polymer, where the loss tangent reaches its maximum at 35 °C then it starts to fall. The addition of cellulose nanocrystals to the polymer positively enhanced both effects. The dramatic drop of storage modulus at 25 °C was less for the nanocomposite, where the drop for the polymer loaded with 15% cellulose nanocrystals was almost cancelled. Similar positive enhancement was found for loss tangent. These enhancements could be attributed to the mechanical coupling effect, in which the NPs connect and form a stiff continuous network linked through hydrogen bonding [ 262 ].
Applications of NPs
NPs, due to their above-mentioned unique or enhanced physicochemical properties, are used in a wide range of applications in different fields. In addition, several potential applications are in research and development. Here we present some examples of these applications.
Applications in medicine and pharma
Metallic and semiconductor NPs have huge potential for cancer diagnosis and therapy based on their enhanced light scattering and absorption properties due to LSPR effect. For instance, Au NPs efficiently absorb light and convert it into localized heat, which can be exploited for selective photothermal therapy of cancer (cancer cell death by heat generated in tumor tissue) [ 263 , 264 ]. In addition, the unique optical properties of Au NPs make them a great candidate for the photodynamic therapy of cancer (the use of a drug that is activated by light to kill cancer cells) [ 265 ]. Gd based NPs have also shown great abilities in tumor growth inhibition [ 266 ], metastasis inhibition [ 267 ], and tumor-specific magnetic resonance contrast enhancement [ 268 ]. Targeted drug delivery is also an important potential application of NPs. ZnO and Fe 3 O 4 NPs were efficiently used for targeted drug delivery and selective destruction of tumor cells [ 269 , 270 , 271 ].
Moreover, NPs have been successfully used in different medical applications such as cellular imaging [ 272 ], or in biosensors for DNA, carbohydrates, proteins, and heavy metal ions [ 273 , 274 ], determination of blood glucose levels [ 275 ], and for medical diagnostics to detect bacteria [ 276 ] and viruses [ 277 ]. For instance, Au NPs were conjugated with SARS-CoV-2 antigens to rapidly detect the presence of SARS-CoV-2 IgM/IgA antibodies in blood samples within 10–15 min [ 278 ], At the same time, due to their antimicrobial and antibacterial activities, NPs such as TiO 2 , ZnO, CuO, and BiVO 4 are being increasing used in various medical products such as catheters [ 279 , 280 ].
Applications in electronics
NPs, due to their novel electronic and optical properties, have a wide range of potential applications in imaging techniques and electronics. For instance, Gd-based NPs can improve the imaging quality and the contrast agent administration dose of magnetic resonance imaging (MRI). The use of Gd 2 O 3 NPs as a contrasting agent was found to be more efficient than the commonly used agent (Gd-DOTA) at the same concentration [ 281 ]. At the same time, GdPO 4 NPs were successfully used for tumor detection using MRI in 1/10 of the dose typically used with Gd-DTPA agent [ 282 ]. Interestingly, NPs also offer the ability to image and track a single molecule, which can reveal important information about cellular processes such as membrane protein organization and interaction with other proteins. For example, Eu 3+ -doped oxide NPs were used to track a single toxin receptor with a localization precision of 30 nm [ 283 ].
Regarding applications in batteries, an important component in lithium-ion batteries is the separators. Their main function is to prevent the physical contact of anode and cathode, and to provide channels for the transport of ions. The commonly used commercial material in battery separators, a polyolefin microporous membrane, suffers from poor electrolyte uptake and poor thermal stability [ 284 ]. Due to the aerogel structure of some NPs (such as ZnO NPs), they are an ideal choice for separator plates in batteries [ 284 ]. This makes the batteries store a significantly higher amount of energy compared to traditional batteries. For lithium-air batteries, using Pt-Au bimetallic NPs strongly enhances oxygen reduction and oxygen evolution reactions [ 285 ]. Moreover, batteries made of nanocrystalline Ni and metal hydrides last longer and require less charging [ 23 ]. In addition to battery applications, several NPs such as CdS and ZnSe are also used in light-emitting diodes (LED) of modern displays to get higher brightness and bigger screens [ 23 , 286 ]. Other NPs such as CdTe NPs are also used in liquid crystal displays (LCDs) [ 287 ]. The addition of a NP layer to LED and LCD enables them to generate more light using the same amount of energy and enhances their lifetime.
Applications in agriculture
NPs have potential to benefit the agriculture field by providing new solutions to current agricultural and environmental problems [ 288 ]. NPs are mainly used in two forms in agriculture, as nanofertilizers and nanopesticides. Chemical fertilizers have poor efficiency due to leaching and volatilization. In these cases, the farmers usually react by using excessive amounts of fertilizers, which increases crops productivity but has an environmental cost [ 288 ]. In contrast, nanofertilizers are compounds that are applied in smaller amounts than regular chemical fertilizers but yet have better efficiencies [ 289 ]. The difference in efficiency comes from the fact that they are able to release the nutrients just when and where they are required by the plants. In that way, they limit the conversion of excess amounts of fertilizer to gaseous forms or from leaking into the ground water [ 290 ]. Several NPs have been employed in the development of fertilizers, including SiO 2 , ZnO, CuO, Fe, and Mg NPs [ 291 , 292 , 293 ]. These nanofertilizers provide the plants with increased nitrogen fixation, improved seed germination, amelioration to drought stress, increased seed weight, and increased photosynthesis ability [ 291 , 292 , 293 ]. The large surface area and small size of these NPs are the main reasons for the better efficiencies of nanofertilizers over conventional fertilizers [ 294 ].
Several NPs have proven antimicrobial, insecticidal, and nematicidal activities, which makes them a promising alternative to chemical pesticides and a potentially cheaper alternative to biopesticides [ 294 ]. For instance, the photocatalytic activity of TiO 2 NPs gives them a potent antimicrobial activity against Xanthomonas perforans , the causing agent of tomato spot disease [ 295 ]. CuO NPs show insecticidal activity against Spodoptera littoralis , known as African cotton leafworm [ 296 ]. Ag NPs show nematicidal activity against Meloidogyne spp. , root-knot nematodes [ 297 ].
Applications in the food industry
NPs, despite toxological concerns, have impactful applications in several food industry-related process such as food production, preservation, and packaging. TiO 2 NPs are a major promising player in this industry. Their photocatalytic antimicrobial activity makes them an interesting material for food packaging [ 298 ]. In addition, they are also used in sensors to detect volatile organic compounds [ 299 ]. Ag NPs are also promising in food packaging due to their antimicrobial activity. They play an important role in reducing the risk of pathogens and extending food shelf-life [ 294 ]. The efficiency of doping Ag and ZnO NPs to degradable and non-degradable packaging materials for meat, bread, fruit, and dairy products was tested against several yeast, molds, aerobic, and anaerobic bacteria [ 300 ]. For instance, polyvinyl chloride doped with Ag NPs was evaluated for packing minced meet at refrigerator temperature (4 °C); the results showed that Ag NPs significantly helped to slow down bacterial growth, increasing the shelf-life of minced meet from 2 to 7 days [ 301 ].
Effects of NPs on biological systems
Although the use of NPs is exponentially growing, their possible toxicological and hazardous impacts to human health and environment cannot be ignored. NPs may get released to the environment during production stages, usage, recycling, or disposal. These NPs may persist in air, soil, water, or biological systems [ 302 ]. NPs can enter the human or animal body though the skin, orally, or via the respiratory tract, and afterwards move to other parts of the body. The exposure to NPs was found to activate proinflammatory cytokines and chemokines with recruitment of inflammatory cells, which impacts the immune system homeostasis and can lead to autoimmune, allergic, or neoplastic diseases [ 302 ]. Moreover, the exposure to ultrafine particles can cause pulmonary, cardiac, and central nervous system diseases [ 303 , 304 , 305 ]. Similarly, NPs can enter plants cells and cause harmful effects [ 306 ]. For instance, the exposure of ZnO and Al NPs was found to cause root growth inhibition in plants [ 307 , 308 ].
Nanoscience and nanotechnology are inherently transdisciplinary fields of science. With new bio-based approaches, there is a need for biologists to understand not only the basic principles of nanoscience, but also the technologies and methods traditionally employed to characterize nanomaterials. We hope that this review can help to inspire new collaborations across different scientific disciplines, by helping biologists to identify the best technologies—and partners—to characterize their nanomaterials. At the same time, we recommend to take potential biological risks of these new materials into careful consideration already during the planning phase of such experiments.
Availability of data and materials
Not applicable.
https://www.etymonline.com/word/nano .
[SOURCE: ISO/TS 80,004‑2:2015, 4.4].
Abbreviations
Atomic force microscopy
Brunauer–Emmett–Teller
Barrett–Joyner–Halenda
Cyclic voltammetry
Dynamic light scattering
Derjaguin–Landau–Verwey–Overbeek
Dynamic mechanical analysis
Derjaguin–Muller–Toporov
UV–vis diffuse reflectance spectroscopy
Differential scanning calorimetry
Differential thermal analysis
Energy-dispersive X-ray spectroscopy
Electron microscopy
Electron paramagnetic resonance spectroscopy
Electron spin resonance spectroscopy
Fourier-transform infrared spectroscopy
High-angle annular dark-field imaging
International Organization for Standardization
Johnson–Kendall–Roberts
Liquid crystal display
Light-emitting diode
Localized surface plasmon resonance
Magnetic force microscopy
Magnetic resonance imaging
Nanoparticles
Nanoparticle tracking analysis
Photoluminescence spectroscopy
Critical radius
Threshold radius for superparamagnetism
Selected area electron diffraction
Scanning electron microscopy
Surface-enhanced Raman spectroscopy
Surface plasmon resonance
Superconducting quantum interference device
Scanning transmission electron microscopy
Scanning tunneling microscopy
Transmission electron microscopy
Thermogravimetry/differential thermal analysis
Thermogravimetric analysis
Transient hot wire
Universal testing machine
Ultraviolet
Ultraviolet–visible spectroscopy
Vibrating-sample magnetometry
X-ray photoelectron spectroscopy
X-ray diffraction analysis
Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17–71.
Article PubMed Google Scholar
Mulvaney P. Nanoscience vs nanotechnology—defining the field. ACS Nano. 2015. https://doi.org/10.1021/acsnano.5b01418 .
Hasan S. A review on nanoparticles: their synthesis and types. Res J Recent Sci. 2015;2277:2502.
Google Scholar
Feynman RP. Plenty of room at the bottom. In: APS annual meeting. 1959.
Tolochko NK. History of nanotechnology (Chapter 1). In: Kharkin V, Bai C, Kapitza S, Awadelkarim OO, editors. Nanoscience and nanotechnologies (vol. 1). ISBN 978-1-78021-531-0. https://www.eolss.net/ebooklib/bookinfo/nanoscience-nanotechnologies.aspx
Walter P, Welcomme E, Hallégot P, Zaluzec NJ, Deeb C, Castaing J, et al. Early use of PbS nanotechnology for an ancient hair dyeing formula. Nano Lett. 2006;6(10):2215–9.
Article CAS PubMed Google Scholar
Barber DJ, Freestone IC. An investigation of the origin of the colour of the Lycurgus Cup by analytical transmission electron microscopy. Archaeometry. 1990;32(1):33–45.
Article Google Scholar
Atwater HA. The promise of plasmonics. Sci Am. 2007;296(4):56–63.
Brill RH, Cahill ND. A red opaque glass from Sardis and some thoughts on red opaques in general. J Glass Stud. 1988;30:16–27. http://www.jstor.org/stable/24190804
Sharon M. History of nanotechnology: from prehistoric to modern times. New Jersey: Wiley; 2019.
Book Google Scholar
Bratovcic A. Different applications of nanomaterials and their impact on the environment. Int J Mater Sci Eng. 2019;5:1–7.
Gajanan K, Tijare SN. Applications of nanomaterials. Mater Today Proc. 2018;5(1):1093–6.
Article CAS Google Scholar
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot. 2012;35:64–70.
Roduner E. Size matters: why nanomaterials are different. Chem Soc Rev. 2006;35(7):583–92.
Lines MG. Nanomaterials for practical functional uses. J Alloys Compd. 2008;449(1–2):242–5.
Gade A, Ingle A, Whiteley C, Rai M. Mycogenic metal nanoparticles: progress and applications. Biotechnol Lett. 2010;32(5):593–600.
Ikhmayies SJ. Characterization of nanomaterials. JOM. 2014;66(1):28–9.
Ashraf MA, Peng W, Zare Y, Rhee KY. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res Lett. 2018;13(1):1–7.
Suttiponparnit K, Jiang J, Sahu M, Suvachittanont S, Charinpanitkul T, Biswas P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res Lett. 2011;6(1):1–8.
Fubini B, Ghiazza M, Fenoglio I. Physico-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology. 2010;4(4):347–63.
Geoffrion LD, Guisbiers G. Quantum confinement: size on the grill! J Phys Chem Solids. 2020;140: 109320.
Kolahalam LA, Viswanath IVK, Diwakar BS, Govindh B, Reddy V, Murthy YLN. Review on nanomaterials: synthesis and applications. Mater Today Proc. 2019;18:2182–90.
Ealia SAM, Saravanakumar MP. A review on the classification, characterisation, synthesis of nanoparticles and their application. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2017. p. 32019.
Machado S, Pacheco JG, Nouws HPA, Albergaria JT, Delerue-Matos C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci Total Environ. 2015;533:76–81.
Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–31.
Pan K, Zhong Q. Organic nanoparticles in foods: fabrication, characterization, and utilization. Annu Rev Food Sci Technol. 2016;7:245–66.
Ng KK, Zheng G. Molecular interactions in organic nanoparticles for phototheranostic applications. Chem Rev. 2015;115(19):11012–42.
Gujrati M, Malamas A, Shin T, Jin E, Sun Y, Lu Z-R. Multifunctional cationic lipid-based nanoparticles facilitate endosomal escape and reduction-triggered cytosolic siRNA release. Mol Pharm. 2014;11(8):2734–44.
Article CAS PubMed PubMed Central Google Scholar
Long CM, Nascarella MA, Valberg PA. Carbon black vs black carbon and other airborne materials containing elemental carbon: physical and chemical distinctions. Environ Pollut. 2013;181:271–86.
Dresselhaus MS, Dresselhaus G, Eklund PC. Fullerenes. J Mater Res. 1993;8(8):2054–97.
Yuan X, Zhang X, Sun L, Wei Y, Wei X. Cellular toxicity and immunological effects of carbon-based nanomaterials. Part Fibre Toxicol. 2019;16(1):1–27.
Lu K-Q, Quan Q, Zhang N, Xu Y-J. Multifarious roles of carbon quantum dots in heterogeneous photocatalysis. J Energy Chem. 2016;25(6):927–35.
Mauter MS, Elimelech M. Environmental applications of carbon-based nanomaterials. Environ Sci Technol. 2008;42(16):5843–59.
Oh W-K, Yoon H, Jang J. Size control of magnetic carbon nanoparticles for drug delivery. Biomaterials. 2010;31(6):1342–8.
Liu M, Zhao F, Zhu D, Duan H, Lv Y, Li L, et al. Ultramicroporous carbon nanoparticles derived from metal–organic framework nanoparticles for high-performance supercapacitors. Mater Chem Phys. 2018;211:234–41.
Chandra S, Das P, Bag S, Laha D, Pramanik P. Synthesis, functionalization and bioimaging applications of highly fluorescent carbon nanoparticles. Nanoscale. 2011;3(4):1533–40.
Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11–23.
Ahlawat J, Asil SM, Barroso GG, Nurunnabi M, Narayan M. Application of carbon nano onions in the biomedical field: recent advances and challenges. Biomater Sci. 2021. https://doi.org/10.1039/D0BM01476A .
Toshima N, Yonezawa T. Bimetallic nanoparticles—novel materials for chemical and physical applications. New J Chem. 1998;22(11):1179–201.
Nascimento MA, Cruz JC, Rodrigues GD, de Oliveira AF, Lopes RP. Synthesis of polymetallic nanoparticles from spent lithium-ion batteries and application in the removal of reactive blue 4 dye. J Clean Prod. 2018;202:264–72.
Mody VV, Siwale R, Singh A, Mody HR. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. 2010;2(4):282.
Fedlheim DL, Foss CA. Metal nanoparticles: synthesis, characterization, and applications. Boca Raton: CRC Press; 2001.
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41(7):2740–79.
Gupta SM, Tripathi M. An overview of commonly used semiconductor nanoparticles in photocatalysis. High Energy Chem. 2012;46(1):1–9.
Sun S, Murray CB, Weller D, Folks L, Moser A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science (80-). 2000;287(5460):1989–92.
Thomas S, Kumar Mishra P, Talegaonkar S. Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharm Des. 2015;21(42):6165–88.
Moreno-Vega A-I, Gomez-Quintero T, Nunez-Anita R-E, Acosta-Torres L-S, Castaño V. Polymeric and ceramic nanoparticles in biomedical applications. J Nanotechnol. 2012. https://doi.org/10.1155/2012/936041 .
D’Amato R, Falconieri M, Gagliardi S, Popovici E, Serra E, Terranova G, et al. Synthesis of ceramic nanoparticles by laser pyrolysis: from research to applications. J Anal Appl Pyrolysis. 2013;104:461–9.
Wu Q, Miao W, Gao H, Hui D. Mechanical properties of nanomaterials: a review. Nanotechnol Rev. 2020;9(1):259–73.
Pithawalla YB, El-Shall MS, Deevi SC, Ström V, Rao KV. Synthesis of magnetic intermetallic FeAl nanoparticles from a non-magnetic bulk alloy. J Phys Chem B. 2001;105(11):2085–90.
Keesom WH. On the deduction of the equation of state from Boltzmann’s entropy principle’. KNAW Proc. 1912;15:240–56.
Debye P. Molecular forces and their electrical interpretation. Phys Zeitschrift. 1921;22:302–8.
CAS Google Scholar
London F. The general theory of molecular forces. Trans Faraday Soc. 1937;33:8b–26.
Guo D, Xie G, Luo J. Mechanical properties of nanoparticles: basics and applications. J Phys D Appl Phys. 2013;47(1):13001.
Missana T, Adell A. On the applicability of DLVO theory to the prediction of clay colloids stability. J Colloid Interface Sci. 2000;230(1):150–6.
Brant J, Lecoanet H, Wiesner MR. Aggregation and deposition characteristics of fullerene nanoparticles in aqueous systems. J Nanoparticle Res. 2005;7(4):545–53.
Tan S, Sherman RL, Ford WT. Nanoscale compression of polymer microspheres by atomic force microscopy. Langmuir. 2004;20(17):7015–20.
Armini S, Vakarelski IU, Whelan CM, Maex K, Higashitani K. nanoscale indentation of polymer and composite polymer−silica core−shell submicrometer particles by atomic force microscopy. Langmuir. 2007;23(4):2007–14.
Savage T, Rao AM. Thermal properties of nanomaterials and nanocomposites. In: Thermal conductivity. Springer; 2004. p. 261–84.
Andrievski RA. Review of thermal stability of nanomaterials. J Mater Sci. 2014;49(4):1449–60.
Qiu L, Zhu N, Feng Y, Michaelides EE, Żyła G, Jing D, et al. A review of recent advances in thermophysical properties at the nanoscale: from solid state to colloids. Phys Rep. 2020;843:1–81.
Shima PD, Philip J, Raj B. Role of microconvection induced by Brownian motion of nanoparticles in the enhanced thermal conductivity of stable nanofluids. Appl Phys Lett. 2009;94(22): 223101.
Syam Sundar L, Sharma KV. Thermal conductivity enhancement of nanoparticles in distilled water. Int J Nanoparticles. 2008;1(1):66–77.
Eastman JA, Choi SUS, Li S, Yu W, Thompson LJ. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl Phys Lett. 2001;78(6):718–20.
Zebarjadi M, Esfarjani K, Shakouri A, Bahk J-H, Bian Z, Zeng G, et al. Effect of nanoparticle scattering on thermoelectric power factor. Appl Phys Lett. 2009;94(20): 202105.
Zeng G, Zide JMO, Kim W, Bowers JE, Gossard AC, Bian Z, et al. Cross-plane Seebeck coefficient of Er As: In Ga As/In Ga Al As superlattices. J Appl Phys. 2007;101(3):34502.
Kim W, Singer SL, Majumdar A, Vashaee D, Bian Z, Shakouri A, et al. Cross-plane lattice and electronic thermal conductivities of Er As: In Ga As∕ In Ga Al As superlattices. Appl Phys Lett. 2006;88(24):242107.
Likhachev VN, Vinogradov GA, Alymov MI. Anomalous heat capacity of nanoparticles. Phys Lett A. 2006;357(3):236–9.
Wang L, Tan Z, Meng S, Liang D, Li G. Enhancement of molar heat capacity of nanostructured Al 2 O 3 . J Nanoparticle Res. 2001;3(5):483–7.
Wang L, Tan Z, Meng S, Druzhinina A, Varushchenko RA, Li G. Heat capacity enhancement and thermodynamic properties of nanostructured amorphous SiO 2 . J Non Cryst Solids. 2001;296(1–2):139–42.
Borel J-P. Thermodynamical size effect and the structure of metallic clusters. Surf Sci. 1981;106(1–3):1–9.
Gülseren O, Ercolessi F, Tosatti E. Premelting of thin wires. Phys Rev B. 1995;51(11):7377.
Shim J-H, Lee B-J, Cho YW. Thermal stability of unsupported gold nanoparticle: a molecular dynamics study. Surf Sci. 2002;512(3):262–8.
Naitabdi A, Ono LK, Behafarid F, Cuenya BR. Thermal stability and segregation processes in self-assembled size-selected Au x Fe1-x nanoparticles deposited on TiO 2 (110): composition effects. J Phys Chem C. 2009;113(4):1433–46.
Mottet C, Rossi G, Baletto F, Ferrando R. Single impurity effect on the melting of nanoclusters. Phys Rev Lett. 2005;95(3):35501.
Cuenya BR. Synthesis and catalytic properties of metal nanoparticles: size, shape, support, composition, and oxidation state effects. Thin Solid Films. 2010;518(12):3127–50.
Nealon GL, Donnio B, Greget R, Kappler J-P, Terazzi E, Gallani J-L. Magnetism in gold nanoparticles. Nanoscale. 2012;4(17):5244–58.
Matthias BT, Clogston AM, Williams HJ, Corenzwit E, Sherwood RC. Ferromagnetism in solid solutions of Scandium and Indium. Phys Rev Lett. 1961;7(1):7.
Matthias BT, Bozorth RM. Ferromagnetism of a zirconium–zinc compound. Phys Rev. 1958;109(2):604.
Acker F, Fisk Z, Smith JL, Huang CY. Enhanced paramagnetism of TiBe2 and ferromagnetic transitions in TiBe2-xCux. J Magn Magn Mater. 1981;22(3):250–6.
Hori H, Teranishi T, Nakae Y, Seino Y, Miyake M, Yamada S. Anomalous magnetic polarization effect of Pd and Au nano-particles. Phys Lett A. 1999;263(4–6):406–10.
McCurrie RA. Ferromagnetic materials: structure and properties. Cambridge: Academic Press; 1994.
Edelstein AS, Cammaratra RC. Nanomaterials: synthesis, properties and applications. Boca Raton: CRC Press; 1998.
Jun Y, Seo J, Cheon J. Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences. Acc Chem Res. 2008;41(2):179–89.
Skumryev V, Stoyanov S, Zhang Y, Hadjipanayis G, Givord D, Nogués J. Beating the superparamagnetic limit with exchange bias. Nature. 2003;423(6942):850–3.
Kolhatkar AG, Jamison AC, Litvinov D, Willson RC, Lee TR. Tuning the magnetic properties of nanoparticles. Int J Mol Sci. 2013;14(8):15977–6009.
Hu M, Butt H-J, Landfester K, Bannwarth MB, Wooh S, Thérien-Aubin H. Shaping the assembly of superparamagnetic nanoparticles. ACS Nano. 2019;13(3):3015–22.
Marghussian V, Marghussian V. Nano-glass ceramics. Amsterdam: Elsevier; 2015.
Kalubowilage M, Janik K, Bossmann SH. Magnetic nanomaterials for magnetically-aided drug delivery and hyperthermia. Appl Sci. 2019;9(14):2927.
Podaru G, Chikan V. Magnetism in nanomaterials: heat and force from colloidal magnetic particles. 2017;
Song Q, Zhang ZJ. Shape control and associated magnetic properties of spinel cobalt ferrite nanocrystals. J Am Chem Soc. 2004;126(19):6164–8.
Salazar-Alvarez G, Qin J, Sepelak V, Bergmann I, Vasilakaki M, Trohidou KN, et al. Cubic versus spherical magnetic nanoparticles: the role of surface anisotropy. J Am Chem Soc. 2008;130(40):13234–9.
Zhen G, Muir BW, Moffat BA, Harbour P, Murray KS, Moubaraki B, et al. Comparative study of the magnetic behavior of spherical and cubic superparamagnetic iron oxide nanoparticles. J Phys Chem C. 2011;115(2):327–34.
Lee W, Kim MG, Choi J, Park J-I, Ko SJ, Oh SJ, et al. Redox-transmetalation process as a generalized synthetic strategy for core-shell magnetic nanoparticles. J Am Chem Soc. 2005;127(46):16090–7.
Park J-I, Cheon J. Synthesis of “solid solution” and “core-shell” type cobalt–platinum magnetic nanoparticles via transmetalation reactions. J Am Chem Soc. 2001;123(24):5743–6.
Lee J-H, Huh Y-M, Jun Y, Seo J, Jang J, Song H-T, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13(1):95–9.
Kumbhakar P, Ray SS, Stepanov AL. Optical properties of nanoparticles and nanocomposites. Hindawi; 2014.
Khlebtsov NG, Dykman LA. Optical properties and biomedical applications of plasmonic nanoparticles. J Quant Spectrosc Radiat Transf. 2010;111(1):1–35.
Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. Washington: ACS Publications; 2003.
Kreibig U, Vollmer M. Theoretical considerations. In: Optical properties of metal clusters. Springer; 1995. p. 13–201.
Duval Malinsky M, Kelly KL, Schatz GC, Van Duyne RP. Nanosphere lithography: effect of substrate on the localized surface plasmon resonance spectrum of silver nanoparticles. J Phys Chem B. 2001;105(12):2343–50.
Jensen TR, Duval ML, Kelly KL, Lazarides AA, Schatz GC, Van Duyne RP. Nanosphere lithography: effect of the external dielectric medium on the surface plasmon resonance spectrum of a periodic array of silver nanoparticles. J Phys Chem B. 1999;103(45):9846–53.
Rajan AR, Vilas V, Rajan A, John A, Philip D. Synthesis of nanostructured CeO2 by chemical and biogenic methods: optical properties and bioactivity. Ceram Int. 2020;46(9):14048–55.
Fu L, Fu Z. Plectranthus amboinicus leaf extract-assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram Int. 2015;41(2):2492–6.
Cuenya BR, Baeck S-H, Jaramillo TF, McFarland EW. Size-and support-dependent electronic and catalytic properties of Au0/Au3+ nanoparticles synthesized from block copolymer micelles. J Am Chem Soc. 2003;125(42):12928–34.
Shaikhutdinov SK, Meyer R, Naschitzki M, Bäumer M, Freund H-J. Size and support effects for CO adsorption on gold model catalysts. Catal Lett. 2003;86(4):211–9.
Lemire C, Meyer R, Shaikhutdinov S, Freund H. Do quantum size effects control CO adsorption on gold nanoparticles? Angew Chem Int Ed. 2004;43(1):118–21.
Ono LK, Sudfeld D, Cuenya BR. In situ gas-phase catalytic properties of TiC-supported size-selected gold nanoparticles synthesized by diblock copolymer encapsulation. Surf Sci. 2006;600(23):5041–50.
Lu Y, Chen W. Size effect of silver nanoclusters on their catalytic activity for oxygen electro-reduction. J Power Sources. 2012;197:107–10.
Shao M, Peles A, Shoemaker K. Electrocatalysis on platinum nanoparticles: particle size effect on oxygen reduction reaction activity. Nano Lett. 2011;11(9):3714–9.
Valden M, Lai X, Goodman DW. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science (80-). 1998;281(5383):1647–50.
Zhang P, Sham TK. X-ray studies of the structure and electronic behavior of alkanethiolate-capped gold nanoparticles: the interplay of size and surface effects. Phys Rev Lett. 2003;90(24): 245502.
Haruta M. Nanoparticulate gold catalysts for low-temperature CO oxidation. ChemInform. 2004. https://doi.org/10.1002/chin.200448226 .
Xu R, Wang D, Zhang J, Li Y. Shape-dependent catalytic activity of silver nanoparticles for the oxidation of styrene. Chem Asian J. 2006;1(6):888–93.
Henry CR. Morphology of supported nanoparticles. Prog Surf Sci. 2005;80(3–4):92–116.
Humbert MP, Murillo LE, Chen JG. Rational design of platinum-based bimetallic catalysts with enhanced hydrogenation activity. ChemPhysChem. 2008;9(9):1262–4.
Toda T, Igarashi H, Uchida H, Watanabe M. Enhancement of the electroreduction of oxygen on Pt alloys with Fe, Ni, and Co. J Electrochem Soc. 1999;146(10):3750.
Igarashi H, Fujino T, Zhu Y, Uchida H, Watanabe M. CO tolerance of Pt alloy electrocatalysts for polymer electrolyte fuel cells and the detoxification mechanism. Phys Chem Chem Phys. 2001;3(3):306–14.
Croy JR, Mostafa S, Hickman L, Heinrich H, Cuenya BR. Bimetallic Pt-Metal catalysts for the decomposition of methanol: effect of secondary metal on the oxidation state, activity, and selectivity of Pt. Appl Catal A Gen. 2008;350(2):207–16.
Liu P, Nørskov JK. Ligand and ensemble effects in adsorption on alloy surfaces. Phys Chem Chem Phys. 2001;3(17):3814–8.
Carlsson AF, Naschitzki M, Bäumer M, Freund H-J. The structure and reactivity of Al 2 O 3 -supported cobalt–palladium particles: a CO-TPD, STM, and XPS study. J Phys Chem B. 2003;107(3):778–85.
Besenbacher F, Chorkendorff I, Clausen BS, Hammer B, Molenbroek AM, Nørskov JK, et al. Design of a surface alloy catalyst for steam reforming. Science. 1998;279(5358):1913–5.
Ono LK, Roldan-Cuenya B. Effect of interparticle interaction on the low temperature oxidation of CO over size-selected Au nanocatalysts supported on ultrathin TiC films. Catal Lett. 2007;113(3):86–94.
Knapp M, Crihan D, Seitsonen AP, Over H. Hydrogen transfer reaction on the surface of an oxide catalyst. J Am Chem Soc. 2005;127(10):3236–7.
Hendriksen BLM, Frenken JWM. CO oxidation on Pt (110): scanning tunneling microscopy inside a high-pressure flow reactor. Phys Rev Lett. 2002;89(4):46101.
Gong X-Q, Raval R, Hu P. General insight into CO oxidation: a density functional theory study of the reaction mechanism on platinum oxides. Phys Rev Lett. 2004;93(10): 106104.
Gong X-Q, Liu Z-P, Raval R, Hu P. A systematic study of CO oxidation on metals and metal oxides: density functional theory calculations. J Am Chem Soc. 2004;126(1):8–9.
Over H, Seitsonen AP. Oxidation of metal surfaces. Science (80-). 2002;297(5589):2003–5.
Yoon B, Häkkinen H, Landman U, Wörz AS, Antonietti J-M, Abbet S, et al. Charging effects on bonding and catalyzed oxidation of CO on Au8 clusters on MgO. Science (80-). 2005;307(5708):403–7.
Laursen S, Linic S. Oxidation catalysis by oxide-supported Au nanostructures: the role of supports and the effect of external conditions. Phys Rev Lett. 2006;97(2):26101.
Rodriguez JA, Wang X, Liu P, Wen W, Hanson JC, Hrbek J, et al. Gold nanoparticles on ceria: importance of O vacancies in the activation of gold. Top Catal. 2007;44(1–2):73–81.
Yan W, Chen B, Mahurin SM, Dai S, Overbury SH. Brookite-supported highly stable gold catalytic system for CO oxidation. Chem Commun. 2004;17:1918–9.
Rodriguez JA, Liu P, Viñes F, Illas F, Takahashi Y, Nakamura K. Dissociation of SO2 on Au/TiC (001): effects of Au–C interactions and charge polarization. Angew Chemie. 2008;120(35):6787–91.
Vladár AE, Hodoroaba V-D. Characterization of nanoparticles by scanning electron microscopy. In: Characterization of nanoparticles. Elsevier; 2020. p. 7–27.
Kano S, Tada T, Majima Y. Nanoparticle characterization based on STM and STS. Chem Soc Rev. 2015;44(4):970–87.
Kumar A, Dixit CK. Methods for characterization of nanoparticles. In: Advances in nanomedicine for the delivery of therapeutic nucleic acids. Elsevier; 2017. p. 43–58.
Kouvaris P, Delimitis A, Zaspalis V, Papadopoulos D, Tsipas SA, Michailidis N. Green synthesis and characterization of silver nanoparticles produced using Arbutus unedo leaf extract. Mater Lett. 2012;76:18–20.
Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009;32(1):79–84.
Hungund BS, Dhulappanavar GR, Ayachit NH. Comparative evaluation of antibacterial activity of silver nanoparticles biosynthesized using fruit juices. J Nanomed Nanotechnol. 2015;6(2):1.
Li Z, Wang Y, Shen J, Liu W, Sun X. The measurement system of nanoparticle size distribution from dynamic light scattering data. Opt Lasers Eng. 2014;56:94–8.
Raval N, Maheshwari R, Kalyane D, Youngren-Ortiz SR, Chougule MB, Tekade RK. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In: Basic fundamentals of drug delivery. Elsevier; 2019. p. 369–400.
Tripathi RM, Gupta RK, Shrivastav A, Singh MP, Shrivastav BR, Singh P. Trichoderma koningii assisted biogenic synthesis of silver nanoparticles and evaluation of their antibacterial activity. Adv Nat Sci Nanosci Nanotechnol. 2013;4(3):35005.
Roy K, Sarkar CK, Ghosh CK. Photocatalytic activity of biogenic silver nanoparticles synthesized using potato ( Solanum tuberosum ) infusion. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;146:286–91.
Soldatova AV, Balakrishnan G, Oyerinde OF, Romano CA, Tebo BM, Spiro TG. Biogenic and synthetic MnO 2 nanoparticles: size and growth probed with absorption and Raman spectroscopies and dynamic light scattering. Environ Sci Technol. 2019;53(8):4185–97.
Filipe V, Hawe A, Jiskoot W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm Res. 2010;27(5):796–810.
Gross J, Sayle S, Karow AR, Bakowsky U, Garidel P. Nanoparticle tracking analysis of particle size and concentration detection in suspensions of polymer and protein samples: influence of experimental and data evaluation parameters. Eur J Pharm Biopharm. 2016;104:30–41.
Rodrigues MC, Rolim WR, Viana MM, Souza TR, Gonçalves F, Tanaka CJ, et al. Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications. J Dent. 2020;96: 103327.
Moreno-Martin G, Pescuma M, Pérez-Corona T, Mozzi F, Madrid Y. Determination of size and mass-and number-based concentration of biogenic SeNPs synthesized by lactic acid bacteria by using a multimethod approach. Anal Chim Acta. 2017;992:34–41.
Naderi M. Surface area: Brunauer–Emmett–Teller (BET). In: Progress in filtration and separation. Elsevier; 2015. p. 585–608.
Balaji S, Mandal BK, Vinod Kumar Reddy L, Sen D. Biogenic ceria nanoparticles (CeO2 NPs) for effective photocatalytic and cytotoxic activity. Bioengineering. 2020;7(1):26.
Article CAS PubMed Central Google Scholar
Sankar S, Sharma SK, Kim DY. Synthesis and characterization of mesoporous SiO 2 nanoparticles synthesized from biogenic rice husk ash for optoelectronic applications. Int J Eng Sci. 2016;17(1):353–8.
Aher YB, Jain GH, Patil GE, Savale AR, Ghotekar SK, Pore DM, et al. Biosynthesis of copper oxide nanoparticles using leaves extract of Leucaena leucocephala L. and their promising upshot against diverse pathogens. Int J Mol Clin Microbiol. 2017;7(1):776–86.
Ghotekar S, Pansambal S, Pawar SP, Pagar T, Oza R, Bangale S. Biological activities of biogenically synthesized fluorescent silver nanoparticles using Acanthospermum hispidum leaves extract. SN Appl Sci. 2019;1(11):1–12.
Bardestani R, Patience GS, Kaliaguine S. Experimental methods in chemical engineering: specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can J Chem Eng. 2019;97(11):2781–91.
Gelb LD, Gubbins KE. Pore size distributions in porous glasses: a computer simulation study. Langmuir. 1999;15(2):305–8.
Epp J. X-ray diffraction (XRD) techniques for materials characterization. In: Materials characterization using nondestructive evaluation (NDE) methods. Elsevier; 2016. p. 81–124.
Hazarika M, Borah D, Bora P, Silva AR, Das P. Biogenic synthesis of palladium nanoparticles and their applications as catalyst and antimicrobial agent. PLoS ONE. 2017;12(9): e0184936.
Groarke R, Vijayaraghavan RK, Powell D, Rennie A, Brabazon D. Powder characterization—methods, standards, and state of the art. In: Fundamentals of laser powder bed fusion of metals. Elsevier; 2021. p. 491–527.
Nasrollahzadeh M, Atarod M, Sajjadi M, Sajadi SM, Issaabadi Z. Plant-mediated green synthesis of nanostructures: mechanisms, characterization, and applications. In: Interface science and technology. Elsevier; 2019. p. 199–322.
Goldstein JI, Newbury DE, Michael JR, Ritchie NWM, Scott JHJ, Joy DC. Scanning electron microscopy and X-ray microanalysis. Cham: Springer; 2017.
Balasubramanian S, Kala SMJ, Pushparaj TL. Biogenic synthesis of gold nanoparticles using Jasminum auriculatum leaf extract and their catalytic, antimicrobial and anticancer activities. J Drug Deliv Sci Technol. 2020;57: 101620.
Khan M, Khan M, Kuniyil M, Adil SF, Al-Warthan A, Alkhathlan HZ, et al. Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalt Trans. 2014;43(24):9026–31.
Barabadi H, Kobarfard F, Vahidi H. Biosynthesis and characterization of biogenic tellurium nanoparticles by using Penicillium chrysogenum PTCC 5031: a novel approach in gold biotechnology. Iran J Pharm Res IJPR. 2018;17(Suppl2):87.
CAS PubMed Google Scholar
Fayaz M, Tiwary CS, Kalaichelvan PT, Venkatesan R. Blue orange light emission from biogenic synthesized silver nanoparticles using Trichoderma viride . Colloids Surf B Biointerfaces. 2010;75(1):175–8.
Otten MT. High-Angle annular dark-field imaging on a tem/stem system. J Electron Microsc Tech. 1991;17(2):221–30.
Utsunomiya S, Ewing RC. Application of high-angle annular dark field scanning transmission electron microscopy, scanning transmission electron microscopy-energy dispersive X-ray spectrometry, and energy-filtered transmission electron microscopy to the characterization of nanopar. Environ Sci Technol. 2003;37(4):786–91.
Haverkamp RG, Marshall AT, van Agterveld D. Pick your carats: nanoparticles of gold–silver–copper alloy produced in vivo. J Nanoparticle Res. 2007;9(4):697–700.
Hossain M, Polash SA, Takikawa M, Shubhra RD, Saha T, Islam Z, et al. Investigation of the antibacterial activity and in vivo cytotoxicity of biogenic silver nanoparticles as potent therapeutics. Front Bioeng Biotechnol. 2019;7:239.
Article PubMed PubMed Central Google Scholar
Kimber RL, Lewis EA, Parmeggiani F, Smith K, Bagshaw H, Starborg T, et al. Biosynthesis and characterization of copper nanoparticles using Shewanella oneidensis : application for click chemistry. Small. 2018;14(10):1703145.
Fadley CS. X-ray photoelectron spectroscopy: progress and perspectives. J Electron Spectros Relat Phenomena. 2010;178:2–32.
Lykhach Y, Kozlov SM, Skála T, Tovt A, Stetsovych V, Tsud N, et al. Counting electrons on supported nanoparticles. Nat Mater. 2016;15(3):284–8.
Sneha K, Sathishkumar M, Lee SY, Bae MA, Yun Y-S. Biosynthesis of Au nanoparticles using cumin seed powder extract. J Nanosci Nanotechnol. 2011;11(2):1811–4.
Aygun A, Gülbagca F, Ozer LY, Ustaoglu B, Altunoglu YC, Baloglu MC, et al. Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J Pharm Biomed Anal. 2020;179: 112961.
Gulbagca F, Ozdemir S, Gulcan M, Sen F. Synthesis and characterization of Rosa canina-mediated biogenic silver nanoparticles for anti-oxidant, antibacterial, antifungal, and DNA cleavage activities. Heliyon. 2019;5(12): e02980.
Huo Y-C, Li W-W, Chen C-B, Li C-X, Zeng R, Lau T-C, et al. Biogenic FeS accelerates reductive dechlorination of carbon tetrachloride by Shewanella putrefaciens CN32. Enzyme Microb Technol. 2016;95:236–41.
Manor J, Feldblum ES, Zanni MT, Arkin IT. Environment polarity in proteins mapped noninvasively by FTIR spectroscopy. J Phys Chem Lett. 2012;3(7):939–44.
Deepty M, Srinivas C, Kumar ER, Mohan NK, Prajapat CL, Rao TVC, et al. XRD, EDX, FTIR and ESR spectroscopic studies of co-precipitated Mn-substituted Zn–ferrite nanoparticles. Ceram Int. 2019;45(6):8037–44.
Chevali V, Kandare E. Rigid biofoam composites as eco-efficient construction materials. In: Biopolymers and biotech admixtures for eco-efficient construction materials. Elsevier; 2016. p. 275–304.
Składanowski M, Golinska P, Rudnicka K, Dahm H, Rai M. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med Microbiol Immunol. 2016;205(6):603–13.
Tugarova AV, Mamchenkova PV, Dyatlova YA, Kamnev AA. FTIR and Raman spectroscopic studies of selenium nanoparticles synthesised by the bacterium Azospirillum thiophilum . Spectrochim Acta Part A Mol Biomol Spectrosc. 2018;192:458–63.
Sikora A, Bartczak D, Geißler D, Kestens V, Roebben G, Ramaye Y, et al. A systematic comparison of different techniques to determine the zeta potential of silica nanoparticles in biological medium. Anal methods. 2015;7(23):9835–43.
Gavade NL, Kadam AN, Suwarnkar MB, Ghodake VP, Garadkar KM. Biogenic synthesis of multi-applicative silver nanoparticles by using Ziziphus jujuba leaf extract. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;136:953–60.
Edison TJI, Sethuraman MG. Biogenic robust synthesis of silver nanoparticles using Punica granatum peel and its application as a green catalyst for the reduction of an anthropogenic pollutant 4-nitrophenol. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;104:262–4.
Ballottin D, Fulaz S, Souza ML, Corio P, Rodrigues AG, Souza AO, et al. Elucidating protein involvement in the stabilization of the biogenic silver nanoparticles. Nanoscale Res Lett. 2016;11(1):1–9.
Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomed Nanotechnol Biol Med. 2010;6(1):103–9.
Menon S, KS SD, Agarwal H, Shanmugam VK. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid Interface Sci Commun. 2019;29:1–8. https://doi.org/10.1016/j.colcom.2018.12.004
Chooto P. Cyclic voltammetry and its applications. In: Voltammetry. IntechOpen; 2019. p. 1.
Saw EN, Grasmik V, Rurainsky C, Epple M, Tschulik K. Electrochemistry at single bimetallic nanoparticles—using nano impacts for sizing and compositional analysis of individual AgAu alloy nanoparticles. Faraday Discuss. 2016;193:327–38.
Testolin A, Cattaneo S, Wang W, Wang D, Pifferi V, Prati L, et al. Cyclic voltammetry characterization of Au, Pd, and AuPd nanoparticles supported on different carbon nanofibers. Surfaces. 2019;2(1):205–15.
Khan AU, Wei Y, Khan ZUH, Tahir K, Khan SU, Ahmad A, et al. Electrochemical and antioxidant properties of biogenic silver nanoparticles. Int J Electrochem Sci. 2015;10(10):7905–16.
Roy N, Mondal S, Laskar RA, Basu S, Mandal D, Begum NA. Biogenic synthesis of Au and Ag nanoparticles by Indian propolis and its constituents. Colloids Surf B Biointerfaces. 2010;76(1):317–25.
Long DA. Raman spectroscopy. New York. 1977;1.
Huang M, Yan H, Chen C, Song D, Heinz TF, Hone J. Phonon softening and crystallographic orientation of strained graphene studied by Raman spectroscopy. Proc Natl Acad Sci. 2009;106(18):7304–8.
Lin T, Song Y-L, Liao J, Liu F, Zeng T-T. Applications of surface-enhanced Raman spectroscopy in detection fields. Nanomedicine. 2020;15(30):2971–89.
Prasad C, Yuvaraja G, Venkateswarlu P. Biogenic synthesis of Fe 3 O 4 magnetic nanoparticles using Pisum sativum peels extract and its effect on magnetic and methyl orange dye degradation studies. J Magn Magn Mater. 2017;424:376–81.
Anghel L, Balasoiu M, Ishchenko LA, Stolyar S V, Kurkin TS, Rogachev A V, et al. Characterization of bio-synthesized nanoparticles produced by Klebsiella oxytoca. In: Journal of Physics: Conference Series. IOP Publishing; 2012. p. 12005.
Lahr RH, Vikesland PJ. Surface-enhanced Raman spectroscopy (SERS) cellular imaging of intracellulary biosynthesized gold nanoparticles. ACS Sustain Chem Eng. 2014;2(7):1599–608.
Skoog DA, Holler FJ, Crouch SR, editors. Principles of instrumental analysis (7th edn). Boston, USA: Cengage learning; 2017. ISBN 978-1-305-57721-3
Patel S, Patel P, Undre SB, Pandya SR, Singh M, Bakshi S. DNA binding and dispersion activities of titanium dioxide nanoparticles with UV/vis spectrophotometry, fluorescence spectroscopy and physicochemical analysis at physiological temperature. J Mol Liq. 2016;213:304–11.
Al-Hakkani MF. Biogenic copper nanoparticles and their applications: a review. SN Appl Sci. 2020;2(3):1–20.
Harne S, Sharma A, Dhaygude M, Joglekar S, Kodam K, Hudlikar M. Novel route for rapid biosynthesis of copper nanoparticles using aqueous extract of Calotropis procera L. latex and their cytotoxicity on tumor cells. Colloids Surf B Biointerfaces. 2012;95:284–8.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Therapeutic Nanoparticles and Their Targeted Delivery Applications
Abuzer alp yetisgin.
1 Materials Science and Nano-Engineering Program, Faculty of Engineering and Natural Sciences, Sabanci University, Istanbul 34956, Turkey; ude.vinuicnabas@play
Sibel Cetinel
2 Nanotechnology Research and Application Center (SUNUM), Sabanci University, Istanbul 34956, Turkey; ude.vinuicnabas@lenitec
Merve Zuvin
3 Mechatronics Engineering Program, Faculty of Engineering and Natural Sciences, Sabanci University, Istanbul 34956, Turkey; ude.vinuicnabas@nivuzevrem (M.Z.); [email protected] (A.K.)
4 Center of Excellence for Functional Surfaces and Interfaces for Nano Diagnostics (EFSUN), Sabanci University, Istanbul 34956, Turkey
Ozlem Kutlu
Nanotechnology offers many advantages in various fields of science. In this regard, nanoparticles are the essential building blocks of nanotechnology. Recent advances in nanotechnology have proven that nanoparticles acquire a great potential in medical applications. Formation of stable interactions with ligands, variability in size and shape, high carrier capacity, and convenience of binding of both hydrophilic and hydrophobic substances make nanoparticles favorable platforms for the target-specific and controlled delivery of micro- and macromolecules in disease therapy. Nanoparticles combined with the therapeutic agents overcome problems associated with conventional therapy; however, some issues like side effects and toxicity are still debated and should be well concerned before their utilization in biological systems. It is therefore important to understand the specific properties of therapeutic nanoparticles and their delivery strategies. Here, we provide an overview on the unique features of nanoparticles in the biological systems. We emphasize on the type of clinically used nanoparticles and their specificity for therapeutic applications, as well as on their current delivery strategies for specific diseases such as cancer, infectious, autoimmune, cardiovascular, neurodegenerative, ocular, and pulmonary diseases. Understanding of the characteristics of nanoparticles and their interactions with the biological environment will enable us to establish novel strategies for the treatment, prevention, and diagnosis in many diseases, particularly untreatable ones.
1. Introduction
Nanomedicine is an emerging approach for the implementation of nanotechnological systems in disease diagnosis and therapy. This branch of nanotechnology can be classified in two main categories: nanodevices and nanomaterials. Nanodevices are miniature devices at nanoscale including microarrays [ 1 , 2 ] and some intelligent machines like respirocytes [ 3 ]. Nanomaterials contain particles smaller than 100 nanometers (nm) in at least one dimension.
Recent explorations of biomedical science resulted in the successful improvement of therapeutic agents’ design in disease treatment. However, there is a major obstacle before the treatment efficiency of various diseases, which is the delivery of therapeutic agents to the target area. The application of conventional therapeutic agents has limitations such as non-selectivity, undesirable side effects, low efficiency, and poor biodistribution [ 4 ]. Therefore, the focus of current research activities is to design well-controlled and multifunctional delivery systems. Association of therapeutic agents with nanoparticles exhibiting unique physicochemical and biological properties and designing their pathways for suitable targeting is a promising approach in delivering a wide range of molecules to certain locations in the body [ 5 ]. This targeted strategy enhances the concentration of therapeutic agent in cells/tissues; thereby, low doses can be used, particularly if there is a contradiction between the therapeutic activity and the toxic effects of the agent. Increasing concentration of therapeutic agents in-target location also improves their therapeutic index by enhancing the efficacy and/or increasing the tolerability in biological systems. Water-insoluble therapeutic agents can also be combined with nanoparticles, which can protect them from physiological barriers and improve their bioavailability. On the other hand, association of therapeutic nanoparticles with contrast agents provides a way of tracking their pathway and imaging their delivery location in in vivo systems.
The aforementioned advantages enable the targeted therapeutic nanoparticles utilization in various fields of medicine. Here, we summarized the physicochemical properties of nanoparticles making them crucial vehicles in nanomedicine and provide a review of the last decade for therapeutic nanoparticles and their targeted delivery applications in various disorders such as cancer and neurodegenerative diseases.
2. Designing Nanoparticles for Therapeutics
Targeted therapy in disease treatment is the approach of delivering appropriate amounts of therapeutic agent for a prolonged period to the affected area within the body. To achieve this, development of safer and more effective therapeutic nanoparticles is crucial and one of the ultimate goals of nanomedicine.
As soon as nanoparticles enter to the bloodstream, they are prone to aggregation and protein opsonization (protein binding to nanoparticle surface as a tag for immune system recognition). The opsonized nanoparticles could be cleared from the bloodstream by phagocytosis or filtration in the liver, spleen, and kidney. This rapid and non-specific clearance by the immune system results in decreased retention time and thus limits bioavailability. By decorating the nanoparticle surface with polyethylene glycol (PEG), carbohydrates, acetyl groups, or protein moieties (arginine-glycine-aspartate (RGD) peptide, albumin), retention time can be altered [ 6 ]. However, such surface modification can also alter the recognition ability for targeted delivery. Thus, the cleanability and biodistribution of therapeutic nanoparticles should be well concerned during the design process.
Size is another important factor playing role in controlling circulation and biodistribution of therapeutic nanoparticles. Nanoparticles smaller than 10 nm, can be easily cleared by physiological systems (filtration through the kidney), while particles larger than 200 nm may be cleared by phagocytic cells in the reticuloendothelial system (RES). Accordingly, therapeutic nanoparticles with a size of ˂100 nm have longer circulation time in the bloodstream. Many studies reported that therapeutic nanoparticles in 20–200 nm size showed a higher accumulation rate in tumors because they cannot be recognized by the RES and filtrated by the kidney [ 7 , 8 , 9 ]. Additionally, blood vessels in tumor areas are higher in number and larger in volume compared to normal tissues. Therefore, nanoparticles with appropriate sizes can reach to the tumor area relatively easily and accumulate for a longer time, which is known as the enhanced permeability and retention (EPR) effect [ 10 , 11 ]. In fact, passive targeting is utilized to accumulate nanoparticles to the tumor site, which is done without functionalizing nanoparticles with targeting moiety. However, in active targeting, the surface of the nanoparticle is coupled with at least one kind of targeting moiety such as, proteins, peptides, nucleic acids, antibodies, or small molecules [ 12 ].
Most of the nanoparticles are taken up within the cells by endocytosis through either clathrin- or caveolae-dependent mechanisms [ 13 ]. The shape of nanoparticles is also critical for biodistribution due to their internalization by the targeted cells. For instance, rod-shaped cationic nanoparticles are easier targets for endosomal uptake than cationic nanoparticles of other shapes, suggesting that these nanoparticles may be comprehended by immune system cells as rod-shaped bacteria [ 14 ].
Surface charge of therapeutic nanoparticles plays an important role in their clearance and targeted delivery. Positively charged nanoparticles generate a higher immune response compared to neutral or negatively charged nanoparticles. Additionally, nanoparticles with a surface potential between −10 and +10 mV are shown to be less susceptible to phagocytosis and non-specific interactions [ 7 , 9 ]. However, the ideal range could depend on the nanoparticle material. Surface charge is also closely related to pH sensibility of nanoparticles. Such nanoparticles can be designed to recognize and locate in specific compartments of the cell. For example, acidic nanoparticles can be targeted to endosomes or lysosomes for releasing their cargo, each of which has a pH of <6.0 [ 8 , 15 , 16 ].
Surface modification of nanoparticles with long-chain polymers such as polyethylene glycol (PEG) was shown to minimize non-specific protein absorption onto the nanoparticle surface. Due to its intrinsic physicochemical properties, PEG is a favorable polymer for therapeutic nanoparticles, which decreases their phagocytic uptake and reduces their accumulation in non-target organs [ 17 ]. Factors such as length, shape, and density of PEG chains affecting surface hydrophilicity and phagocytosis, should be considered before PEGylation of therapeutic nanoparticles. Conjugation of targeting ligands to the surface of PEGylated nanoparticles can improve the target-specific delivery of nanoparticles; however, it also affects their biodistribution.
3. Types of Therapeutic Nanoparticles
Nanomaterials can be classified in two main categories: Nano-structured and nanocrystalline. Nano-structured materials can further be categorized into polymer-based, non-polymeric, and lipid-based nanoparticles. Polymer-based nanoparticles include dendrimers, nanoparticles, micelles, nanogels, protein nanoparticles, and drug conjugates. Non-polymeric nanoparticles include carbon nanotubes, nanodiamonds, metallic nanoparticles, quantum dots, and silica-based nanoparticles. Lipid-based nanoparticles can be divided into liposomes and solid lipid nanoparticles. So far, the majority of the nanoparticles clinically approved for therapeutic use are polymer-based or lipid-based components. Apart from polymer-based, non-polymeric, or lipid-based nano-structured particles, nanocrystalline particles that are formed by the combination of therapeutic agents in crystalline form are also used in some clinical applications ( Figure 1 and Figure 2 ). In this section, we summarized different types of clinically used nanoparticles and their specificity for therapeutic applications, as well as their current delivery strategies in challenging pathophysiological conditions.

Elements of nanotechnology, which are utilized in therapeutic applications.

Schematic representations of the therapeutic nanoparticles.
3.1. Nano-Structured Particles
3.1.1. polymer-based particles.
Dendrimers are widely used polymers in clinical applications due to their hyperbranched, compartmentalized structure, and high monodispersity. Controlling the number of branches in these polymer-based nanoparticles allows their fabrication in very small sizes (1–5 nm). They can be fabricated by polymerization in spherical shape, which leads to the formation of cavities within the dendrimer molecule. Thus, high entrapment efficiency is obtained with high-generation dendrimers, e.g. dendrimers containing more than 64 surface groups, when compared to smaller dendrimers and these are used for the delivery of therapeutic agents. Additionally, dendrimers contain free end groups, which can be easily modified/used for the conjugation of biocompatible compounds to enhance low cytotoxicity and high bio-permeability of the molecule. Such surface modifications can also be applied to improve the target-specific delivery of therapeutic agents. Assembling dendrimers by either encapsulation or complexation makes them attractive vehicles for the concomitant delivery of biologically active molecules such as vaccines, drugs, and genes to the target locations. Currently, mono- or copolymers, such as polyethyleneimine, polyamidoamine, poly(propyleneimine), chitin, etc., are used for therapeutic applications in the form of dendrimers [ 18 , 19 ].
Nanoparticles
Polymer-based nanoparticles, synthetic or natural, provide an alternative way for therapeutic applications due to certain characteristics, such as biocompatibility, non-immunogenicity, non-toxicity, and biodegradability [ 20 ]. In order to decrease the immunogenicity and toxicity of synthetic polymers, like polycaprolactone (PCL), polylactic acid (PLA), and their monomers, the polyester forms are used. On the other hand, natural polymer-based nanoparticles such as chitosan, gelatin, albumin, and alginate seem to overcome toxicity issues and provide significant improvement in the efficiency of therapeutic agents compared to conventional methods. Polymeric nanoparticles are considered as the matrix system, in which the matrix is uniformly dispersed. They can be classified as nanocapsules or nanospheres depending on their composition. In nanocapsules, a unique polymer membrane encloses therapeutic agents, whereas the therapeutic agents are directly dispersed throughout or within the polymer matrix in nanospheres [ 21 ]. Existence of a multitude of preparation methods of polymeric nanoparticles can control the release characteristics of incorporated therapeutic agents, which allows the delivery of a higher concentration of agents to the target location. Moreover, the surface of polymeric nanoparticles could be easily modified and functionalized with a specific recognition ligand which increases the specificity of therapeutic agents in targeted tissue.
Polymeric micelles are mostly used for the systemic delivery of water-insoluble therapeutic agents. They are in ˂100 nm size and formed in solution as aggregates. The component molecules of polymeric micelles are arranged in spheroidal structure, in which a mantle of hydrophilic groups surrounds hydrophobic cores. The existence of hydrophilic surface contributes to their protection from nonspecific uptake by the reticuloendothelial system ensuring their high stability within physiological systems. On the other hand, the hydrophobic core of polymeric micelles can physically trap the water-insoluble, hydrophobic therapeutic agents. The component molecules can also be covalently linked to this hydrophobic core. Consequently, the dynamic structure of polymeric micelles provides a prominent delivery system for therapeutic agents, which allows versatile loading capacity, conjugation of targeted ligands, and lower rate of dissolution [ 22 ].
Drug Conjugates
Conjugation of polymers with drug molecules is generally used for low molecular weight agents, particularly in cancer treatment. This conjugation increases the overall molecular weight of drugs, which induces the pharmacokinetic disposition in the cells. Polymer-drug conjugates serve as carriers with high solubility and stability and promote an EPR effect in cancer cells [ 23 ]. Covalently conjugated polymer-drugs are shown to be more reliable for sustained drug release and enhanced drug capacity [ 24 ]. There are pH-sensitive polymeric drug conjugates, which are made by utilizing pH-responsive chemical bonds between polymer and drug. Thus, the pH sensitivity of the nanoparticle is used for controlled drug release in the tumor site due to its acidic environment [ 25 , 26 ]. It is also reported that polymeric drug conjugates increase the bioavailability of the drug, for instance, shown with paclitaxel and doxorubicin combination therapy [ 24 , 27 , 28 ].
Protein Nanoparticles
Viruses are very efficient and natural carrier systems for transferring their genetic material, which is encapsulated by the capsid proteins. Virus-like particles (VLP), a type of protein nanoparticles, are defined as nano-carrier systems, which have a morphologically similar, virus-isolated structure but do not include the viral genetic material [ 29 , 30 ]. Additionally, caged proteins (CP) are defined as self-assembled protein nanostructures, which are morphologically similar to viruses, however, not deriving from viruses. The VLPs and CPs are attractive nano-carrier systems for the development of the vaccines for cancer because they can induce antigen-specific immune responses against cancer cells [ 31 ]. Moreover, there are protein nanoparticles made by self-assembly of protein polymers, which are isolated proteins from animal or plant origin such as collagen, gelatin, silk, albumin, elastin, and soy. Through genetic engineering, protein polymers are self-assembled into functional drug delivery carriers with advantages of polymer-based nanoparticles [ 32 , 33 ]. Abraxane ® is an FDA-approved protein nanoparticle drug, enabling paclitaxel delivery by albumin. On the other hand, an HIV vaccine made from VLPs led to critical developments, which accelerated research on protein nanoparticles for clinical use [ 34 ].
The gels are defined as non-fluid colloidal or polymeric networks that swell when in contact with fluid. A nanogel is considered as a particle of gel with similar properties, however, with a diameter of less than 100 nm by the International Union for Pure and Applied Chemistry (IUPAC) [ 35 ]. The swelling property with flexible size and high water content of the nanogels is the result of physically or chemically cross-linked natural or synthetic polymers [ 36 , 37 ]. The first reported nanogel was prepared by physical cross-linking of amphiphilic polysaccharides, where cholesterol-bearing pullulans are self-assembled (by hydrophobic interactions) into nanogels in water [ 36 , 38 ]. The nanogels have some advantages compared to other nano-carrier systems such as decreased untimely drug leakage, encapsulating various therapeutic molecules in the same formulation, and easy administration through parental or mucosal routes. The nanogels are used in various applications, including biosensors, biochemical separation, cell culture, bio-catalysis, drug delivery, antitumor therapy, and so on. Among these, delivery of therapeutics such as nucleic acids, vaccines, cytokines, and nasal vaccines are the most widely studied applications of nanogels [ 36 , 37 , 39 ].
3.1.2. Non-Polymeric Particles
Carbon nanotubes.
Carbon nanotubes are carbon-based tubular structures 1 nm in diameter and 1–100 nm in length [ 40 ]. These structures can be obtained by wrapping a single layer of graphite called graphene into a seamless cylinder. The configuration of carbon nanotubes includes single-walled nanotubes (SWNTs), multi-walled nanotubes (MWNTs), and C60 fullerenes. The size and stable geometric shape of carbon nanotubes make them an attractive non-polymeric carrier for therapeutic agents. Particularly, SWNTs and C60 fullerenes have internal diameters of 1–2 nm, which is equivalent to about half of the average DNA helix diameter [ 41 ]. The SWNTs and MWNTs can enter the cell by endocytosis or by direct insertion through the cell membrane. Fullerenes differ in the arrangement of their graphite cylinders and the presence of a high number of conjugated double bonds in their core structure. Experiments with fullerenes have shown that they can be used for the delivery of therapeutics like antibiotics and antiviral and anti-cancer agents [ 42 , 43 , 44 , 45 ]. Additionally, they can protect the injured mitochondria by providing free radicals [ 46 ]. This feature allows for the tissue-selective targeting of mitochondria that can be used for delivering therapeutic agents [ 47 ].
Nanodiamonds (NDs)
Nanodiamonds (NDs) are members of carbon-based nanomaterials with a diameter of less than 100 nm and different shapes with two types of discrete facets, which are generated from various methods, such as the detonation, chemical vapor deposition (CVD), and high-pressure/high-temperature methods [ 48 , 49 ]. The oldest and most commonly used ND preparation is the detonation method, in which NDs are produced by activating a controlled explosion on carbon-containing precursors in a closed chamber. NDs that are prepared by this method usually contain sp 2 carbon on the surface, and the electrostatic potential of surface is dependent on shape and structure of the NDs [ 50 , 51 , 52 ]. The CVD method is preferable for depositing NDs onto various substrates as thin films. The produced ND thin films are of high quality with low defects [ 53 ].
NDs have unique properties, including surface electrostatic properties, low cytotoxicity by a chemically inert core, and low photo-bleaching by the addition of nitrogen defects and can be functionalized by immobilization of various types of biomolecules, which make them remarkable for biomedical applications such as magnetic resonance imaging (MRI), synthesis of contact lenses, and drug delivery for cancer therapy. NDs can be coupled with gadolinium [Gd] (III) as a contrast agent for MRI, and the signal generated from this complex is several times higher compared to Gd (III)-based contrast agents [ 48 , 49 , 50 ].
Metallic Nanoparticles
Metallic nanoparticles used in medical applications are 1–100 nm in size and mostly made up of cobalt, nickel, iron, gold, and their respective oxides like magnetite, maghemite, cobalt ferrite, and chromium dioxide. They can be synthesized and modified with versatile functional chemical groups, which allows them to be decorated with various molecules including therapeutic agents, biological molecules like peptides, proteins, and DNA. As a carrier, they provide unique characteristics such as magnetic properties besides stability and biocompatibility. Thus, magnetic nanoparticles can be targeted to a specific location in the body by using an external magnetic field. Magnetic susceptibility, defined as the ratio of induced magnetization to the applied field, is an important parameter for their medical use. For example, super-paramagnetic iron oxide nanoparticles (SPIONs) have a large magnetic susceptibility, and thus, they are widely used in clinics as contrast agents for magnetic resonance imaging [ 54 ]. Likewise, super-paramagnetic properties facilitate the stable delivery of therapeutic agents to the body/cell and proper accumulation at the target tissue providing a reproducible and safe treatment approach [ 41 , 55 ]. When metallic nanoparticles are subjected to an alternating magnetic field, they can produce heat that is called magnetic hyperthermia, which enables their use in the ablation of tumors for cancer treatment [ 56 , 57 ].
Gold nanoparticles (AuNP) are widely used metallic nanoparticles, especially in cancer diagnosis and therapy. The reason for this is the unique optical and localized surface plasmon resonance (LSPR) and relatively low cytotoxicity due to the inert nature of gold. When the light with appropriate wavelength is administered to AuNPs as external stimuli, due to the LSPR property, they exhibit photothermal conversion and heat up the targeted tumor tissue to kill cancer cells. Besides, AuNPs are used for drug delivery, where (at the targeted site) the light irradiation can trigger the drug release [ 58 , 59 ]. Moreover, AuNPs’ optical and LSPR properties can be tailored for applications such as imaging, optical and electrochemical detection, diagnosis, and photothermal therapy [ 60 , 61 ].
Quantum Dots
Quantum dots (QDs) are tiny particles or nanocrystals of a semiconducting material with diameters in the range of 2-10 nm. These particles consist of a semiconductor inorganic core such as CdSe and an aqueous organic coated shell such as ZnS [ 62 ]. QDs produce distinctive fluorescence colors that are partly the result of unusually high surface-to-volume ratios for such particles. The core structure of QDs determines the color emitted, while the outer aqueous shell can be used for conjugation of biomolecules such as peptides, protein, or DNA [ 63 ]. QDs can also carry a cap, which improves their solubility in aqueous buffers. Due to their narrow emission, bright fluorescence, and high photo-stability, QDs can be used for tracking therapeutic agents within the cells/tissues [ 64 , 65 ]. Although the medical use of QDs is still debated, their surfaces for versatile bioconjugation, adaptable photophysical properties for multiplexed detection, and superior stability for longer investigation periods make them a superior candidate than other fluorescence agents.
Silica-Based Nanoparticles
Silica-based nanoparticles offer considerable advantages in nanotechnology due to their applicability for designing complex systems and cost-effectiveness. Their specific surface characteristics, porosity, and capacity for functionalization make them attractive tools for therapeutic delivery [ 66 ]. Silica nanoparticles have a large surface area covered with polar silanol groups, which are favorable for water adsorption and improve the stability of therapeutic agents. In addition, silica-based nanoparticles have ability to interact with nucleic acids, which allows their use as targeted delivery vehicles [ 67 ]. Their nanopore size and density can be tailored to achieve a constant delivery rate. Moreover, encapsulation of therapeutic agents within silica-based nanoparticles provides solid media for the delivery of agents. Pores of silica nanoparticles can be capped with various stimuli-responsive molecules to increase the rate of drug release in the targeted tissue. For instance, mesoporous silica nanoparticles capped with β-cyclodextrin were developed to release the encapsulated drug at the acidic tumor tissue [ 58 ]. Combination of these nanoparticles with contrast agents such as gold, silver, iron oxide, organic dyes, and quantum dots facilitates their tracking in biological systems [ 68 ]. Furthermore, these nanoparticles are used as additives in pharmaceutical production to improve the mechanical properties and the biocompatibility of the product.
3.1.3. Lipid-Based Nanoparticles
Liposomes are vesicles synthesized through the hydration of dry phospholipids. They can be prepared in distinct structure, composition, size, and flexibility with a variety of lipid molecules and further surface modification. One of the most important advantages of liposomes is their ability to fuse with cell membrane and release their contents into the cytoplasm, which makes them suitable intelligent carrier systems for targeted delivery. The simplest liposome is composed of a lipid bilayer surrounding a hollow core with a diameter of 50–1000 nm. The therapeutic molecules can be loaded into this hollow core for delivery [ 69 , 70 , 71 ]. Depending on the number of bilayers, they are classified into three basic types: multilamellar, small unilamellar, and large unilamellar. Multilamellar vesicles consist of several lipid bilayers separated from one another by aqueous spaces. In contrast, unilamellar vesicles consist of a single bilayer surrounding the entrapped aqueous space. These structural properties allow them to carry both hydrophobic and hydrophilic molecules. Hydrophilic molecules can be carried in the aqueous interior of the liposome, while hydrophobic molecules can be dissolved in the lipid membrane [ 72 ]. Additionally, more than one type of drug can be loaded either within two compartments (lipid and aqueous) or several aqueous layers of multilamellar liposomes. This also allows different drug molecules to be released in sequence with dissociation of layers from the outer shell to the inner core [ 73 ]. The neutral or positively charged small liposomes have higher circulation time when compared to large, unmodified liposomes [ 74 ]. Moreover, surface modifications can be obtained by either coating it with a functionalized polymer or PEG chains that improve targeted delivery and increase their circulation time in biological systems [ 75 ]. The liposomes are investigated for a wide variety of therapeutic applications, such as cancer diagnostic and therapy, vaccines, brain-targeted drug delivery, and anti-microbial therapy [ 71 ].
Exosomes are naturally formed and secreted by various types of cells. They are endosome-derived extracellular vesicles with a size of 30–150 nm and usually exist in different body fluids such as saliva, blood, urine, and breast milk [ 76 ]. Exosomes are cell membrane-like lipid bilayer vesicles, which contain various substances, including RNA, DNA, glycolipids, and proteins [ 77 , 78 ]. Exosomes play an important role in intracellular communication by transferring various compounds in physiological mechanisms such as immune response, neural communication, antigen presentation in diseases like cancer, cardiovascular disease, diabetes, and inflammation [ 76 , 79 ]. Since these vesicles can be isolated from a patient’s bodily fluids, allogenic exosomes have an advantage over the immune system, which can easily protect the cargo from rapid clearance and improve the drug delivery to targeted sites [ 80 ]. Therefore, studies on the potential of exosomes for the use as drug delivery carriers for cancer and autoimmune diseases, diagnostic biomarkers of cancer, and even for tissue regeneration are emerging [ 81 , 82 ].
Solid Lipid Nanoparticles (SLN)
Solid lipid nanoparticles (SLN) are aqueous colloidal dispersions comprised of a lipid matrix that is solid at room and body temperatures. Surfactants improve their stability, whereas the choice of lipid affects the drug delivery characteristics. The size of SLNs varies from 10 to 1000 nm depending on the production approach [ 83 ]. SLNs as a sub-category of lipid carriers can encapsulate very high amounts of lipophilic drugs as well as hydrophilic drugs and nucleic acids, making them versatile drug delivery vehicles [ 84 , 85 ]. SLNs can be decorated or loaded with various moieties, including antibodies, magnetic nanoparticles, pH sensitive lipids/polymers to modulate targeted delivery, and stimuli-responsive drug release [ 86 , 87 ]. They are shown to be effective carriers for cancer, pulmonary, and oral drug delivery purposes [ 88 , 89 ].
3.2. Nanocrystalline Particles
Nanocrystalline particles, or nanocrystals, are carrier-free drug particles with a crystallite size of only a few nanometers. Nanocrystal formulations are widely prepared for poorly water-soluble drugs suffering from limited bioavailability and absorption as a highly cost-effective approach. Generally, the size reduction is a suitable way to enhance the bioavailability of agents, where the dissolution velocity is the rate-limiting step. The crystalline structure leads to an increased overall surface area and thus increases dissolution velocity. This characteristic improves the solubility, which is important especially when the therapeutic index of the agent is limited due to absorption problems. Relatively, nanocrystalline particles enable the quick absorption of therapeutic agents due to their fast dissolution, offering an advantage for agents that need to work fast. By modifying the nanocrystal surface, it is possible to achieve a prolonged or targeted release, allowing for the use of therapeutic agents in low doses and decreasing side effects [ 90 ].
4. Targeted Delivery Applications of Therapeutic Nanoparticles
Targeted delivery refers to the successful direction of therapeutic agent and its dominant accumulation within a desirable site. For the efficient targeted delivery, the agent-loaded system should be retained in the physiological system for the preferable time, evade the immunological system, target specific cell/tissue, and release the loaded therapeutic agent [ 91 ]. Currently, targeted delivery of nanoparticles is widely studied in cancer treatment. Over 20% of the therapeutic nanoparticles already in clinics or under clinical evaluation were developed for anti-cancer applications. In addition, related research has focused on nanoparticle-mediated therapy for some other diseases, such as neurodegenerative, infectious, autoimmune, etc. diseases. The subsequent section provides up-to-date applications of therapeutic nanoparticles as targeted delivery systems in several diseases, and Table 1 summarizes the Food and Drug Administration (FDA)- and the European Medicines Agency (EMA)-approved nano-drug formulations since 2009.
Food and Drug Administration (FDA)- and European Medicines Agency (EMA)-approved therapeutic nanoparticles since 2009.
4.1. Cancer
Cancer is one of the major causes of death, and chemotherapy is widely used as a treatment approach for various cancer types. However, chemotherapeutic agents suffer from the lack of aqueous solubility, exhibit dose-dependent toxicity, and their tumor specificity is inadequate [ 108 ]. Multidrug resistance is another challenge in chemotherapy, which mainly occurs due to increased efflux pumps that are responsible for the export of anti-cancer agents from cell membrane [ 109 ].
Recent developments of nano-delivery systems overcome these limitations targeting directly the cancer cell, delivering the agent at a controlled rate, and optimizing the therapeutic efficacy [ 110 ]. A variety of nanoparticles has been developed for delivery of anti-cancer agents, and two major mechanisms are used to deliver them at the tumor site: Passive targeting and active targeting [ 111 ]. Passive targeting is based on the accumulation of therapeutic agent in the tumors due to their distinctive features compared to normal tissues. Tumors have leaky vasculature and defective lymphatic drainage promoting the delivery and retention of therapeutic nanoparticles, commonly referred to as the EPR effect [ 112 ]. Nonetheless, nanoparticles encounter several obstacles during passive targeting. Mucosal barriers or non-specific uptake of particles on the way to their target limit their efficiency. In contrast, active targeting achieves selective recognition of the targeted cells by carrying ligands at the surface of nanoparticles that bind to receptors or stimuli-based carriers [ 113 , 114 ].
Currently, the majority of FDA-approved therapeutic nanoparticles are developed as a re-formulation of chemotherapeutic drugs combinations with polymeric nanoparticles. The first nano-drug for cancer treatment was a PEGylated liposomal formulation of doxorubicin (Doxil ® , Caelyx ® ). Doxil is formulated with sterically stabilized liposomes composed of phospholipids, cholesterol, and a lipopolymer (PEG) to achieve extended circulation time and eliminate RES. It is less than 120 nm and thus takes the advantage of EPR in accumulation in the tumor and in resulting in decreased cardiotoxicity [ 115 ]. Another example of liposomal drug formulation is Marqibo ® , which is approved by the FDA for the treatment of acute lymphocytic leukemia in adults [ 92 ]. The liposomal formulation of Marqibo ® (Vincristine sulfate) is generated from sphingomyelin and cholesterol, which greatly improves the circulation time and expedite dose intensification as compared to standard Vincristine [ 116 ]. There are several lipid-based formulations under clinical trials as well [ 117 , 118 ]. For instance, one of the first line cancer chemotherapy agents, cisplatin, is formulated in soy phosphatidylcholine (SPC-3), cholesterol, dipalmitoyl phosphatidyl glycerol (DPPG), and methoxy-PEG-distearoyl phosphatidyl ethanolamine (mPEG2000-DSPE) under the brand name Lipoplatin ® (Regulon, Inc.). Lipoplatin ® pre-clinical trials revealed less nephrotoxicity in comparison to the conventional drug. Consequently, The European Medicines Agency (EMA) announced Lipoplatin ® as an orphan drug for the treatment of pancreatic adenocarcinoma, breast cancer, and advanced gastric cancer [ 119 , 120 , 121 , 122 ].
Drug conjugates are arranged to facilitate either the targeted delivery of cytotoxic drugs to tumor cells or decreased systemic cytotoxicity of the drug [ 123 ]. The FDA approved such an antibody-drug conjugate namely Kadcyla ® for the treatment of HER2+ breast cancer. The drug Kadcyla ® is formed by covalent conjugation of maytansine derivative DM1 to transtuzumab (known as Herceptin ® ) through the lysine residues of the antibody [ 124 , 125 ]. Thus, DM1 is delivered to HER2+ breast cancer cells through transtuzumab recognition of HER receptor, and internalized DM1 triggers apoptosis. The albumin-conjugated nanoparticle version of the anti-cancer drug paclitaxel (Abraxane ® ) or a re-formulation of the rapamycin drug with micellar nanoparticles (Rapamune ® ) are other FDA-approved therapeutic nanoparticles with lower side effects and improved therapeutic indices over their conventional counterparts [ 126 ]. Table 2 summarizes the therapeutic nanoparticles evaluated in the pre-clinical or clinical trials.
Therapeutic nanoparticles under pre-clinical or clinical evaluation.
4.2. Infectious Diseases
The major therapeutic approach for infectious disease is the use of anti-microbial drugs. However, pathogens can become resistant, where anti-microbial drugs become therapeutically insufficient. In that case, administration dose and frequency are increased, which results in increased side effects and toxicity. Besides, many pathogens are located intracellularly in an active or latent state, which prevents the access of anti-microbial drugs [ 146 , 147 ]. The use of nano-delivery systems promises to overcome such limitations. Therefore, there is a growing interest to generate nanotechnology-based formulations against various pathogens, such as bacteria, viruses, fungi, or parasites.
Application of nano-delivery for the treatment of infectious diseases includes both polymer-based and non-polymeric nanoparticles, as well as liposomes that improve the anti-microbial activity of drugs [ 148 ]. Ciprofloxacin is a broad-spectrum antibiotic prescribed for lung infections. Lipoquin™, the liposome formulation of ciprofloxacin, is designed as an inhaled formula for sustained release of up to 24 h and thus eliminated the systemic effects of the high-dose antibiotic [ 149 ]. Similarly, the anti-fungal liposomal carrier Ambisome ® (amphotericin B) is designed to reduce associated toxicity of amphotericin B [ 150 ]. Due to its low systemic toxicity, liposomal amphotericin B can be used for highly immunocompromised patients with HIV infection or disseminated histoplasmosis [ 151 ].
Nanoparticles can also be fabricated into anti-viral drugs. Virosomal vaccines are the carriers of viral adjuvants such as viral glycoproteins, toll-like receptor, or protein fractions. Such vaccines, e.g., Inflexal ® V for hepatitis-A and Epaxal ® for influenza, are already used in clinics for therapeutic purposes [ 152 ]. Virosomal vaccine formulations are also being investigated, for instance in clinical trials for Ebola virus (EBOV). That approach creates safer viral vaccines and induces strong and long-lasting immune response.
Anti-microbial nano-drugs are also used to decorate medical devices to eliminate biofilm formation [ 153 ], such as AgNPs in central venous catheters [ 154 ]. Furthermore, there are some nanoparticles used in diagnosis or as medical devices like Verigene ® , Silverline ® , Acticoat™, or Endorem™ SPIONs [ 105 , 155 , 156 ].
A short list of therapeutic nanoparticles against resistant strains and some nano-delivery systems used for prevention and treatment against bacterial infection can be found in Table 3 .
Therapeutic nanoparticles and nano-delivery systems for the prevention and treatment of bacterial infections.
4.3. Autoimmune Diseases
Rheumatoid arthritis (RA) and acquired immunodeficiency syndrome (AIDS) are the main two diseases being treated using nano-delivery systems.
RA is one of the common and severe autoimmune diseases affecting almost 1% of the world’s population. Despite the cause being unknown, the complex interaction between immune mediators is likely responsible for the bone and cartilage destruction. New therapy approaches are able to improve the quality of the patient’s life; however, a restricted administration route and the requirement of repetitive long-term treatment result in systemic adverse effects [ 171 ]. Nanoparticle systems are promising for the delivery of therapeutic agents particularly to target inflamed tissue (synovial membrane), thereby preventing systemic and undesired effects. Certolizumab pegol (CZP) is a TNF-α inhibitor widely used in clinic [ 161 , 172 ]. Nano-formulation of CZP with PEG increases its half-life to ∼14 days, and clinical trials have shown promising results for the long-term treatment on RA patients [ 173 ]. Targeting inflamed tissues by using stand-alone C60 fullerenes (non-drug loaded) showed promising results in RA treatment by reducing synovitis and alleviated bone resorption and destruction [ 174 ].
Acquired immunodeficiency syndrome (AIDS) is another autoimmune disease lacking treatment. Current clinical therapy is called highly active anti-retroviral treatment (HAART), which consists of a combination of at least three anti-HIV medications suppressing human immunodeficiency virus (HIV) replication. Although this therapeutic approach has contributed to a decreased mortality rate, it is not fully effective [ 175 ]. Recently, nano-delivery systems are under development based on polymeric and liposomal nano-carriers to provide a target-specific and sustained release formulation of anti-HIV drugs. The goal is to improve efficiency of anti-HIV treatment and limit systemic side effects [ 176 ]. For instance, efavirenz is loaded into poly(propyleneimine) dendrimers (TuPPI), which are decorated with Tuftsin. Final TuPPI particles were able to recognize mononuclear phagocytic cells through Tuftsin and resulted in significantly higher uptake in HIV infected macrophages compared to uninfected cells [ 177 ]. Additional examples of nanoparticle drug formulations for AIDS therapy are summarized in Table 4 .
Therapeutic nanoparticle drug formulations for the treatment of AIDS disease.
4.4. Cardiovascular Diseases
Cardiovascular disease (CVD) affects the cardiovascular system, vascular systems of the brain and kidney, and peripheral arteries. Despite many novel therapeutic strategies such as gene delivery and cell transplantation, heart failure is still a leading cause of mortality worldwide [ 186 ]. Utilization of nanoparticle-based formulations to treat cardiovascular diseases is mostly focused on targeted delivery and increasing bioavailability for vascular restenosis.
As a nanoparticle drug for restenosis, liposomes formed by phosphatidylcholine and cholesterol were loaded with small drug sirolimus and coated with chitosan. The resulted liposomal sirolimus is shown to significantly inhibit vascular restenosis [ 187 ]. Another drug, carvedilol is a β-blocker widely used for the treatment of hypertension, myocardial infarction (MI), congestive heart failure, and post-MI left ventricular dysfunction. However, its low water solubility and extensive pre-systemic metabolism limit its bioavailability. The niosome-based nanoparticle formulation encapsulating carvedilol reached ~1.7–2.3-fold higher plasma concentrations compared to the free drug, resulting in enhanced bioavailability and improved therapeutic effect [ 188 ]. Similarly, resveratrol is a cardio-protective polyphenol with low bioavailability and water-solubility. Its nano-formulations both as solid lipid nanoparticle and liposome showed enhanced oral bioavailability and controlled release [ 189 ].
Angiogenic therapy of myocardial ischemia with vascular endothelial growth factor (VEGF) is a convenient approach to overcome hypoxia-dependent side effects. Polymeric particles loaded with VEGF have been proposed as a promising system to improve vasculogenesis and tissue remodeling in an acute myocardial ischemic model [ 190 , 191 ]. A targeting nano-delivery system in atherosclerosis is also achieved to visualize and treat atherosclerotic lesions by using magneto-fluorescent nanoparticles or ligand-binding polymeric micelles [ 192 ].
4.5. Neurodegenerative Diseases
Neurodegenerative diseases (NDs) are characterized via the progressive loss of the function of neurons, which subsequently causes neuronal death. Patients with NDs, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS), have symptoms related to movement, memory, and dementia as a result of gradual loss of neurons. Although significant progress has been achieved in the treatment of NDs, the therapeutic strategies are limited due to the restrictive structure of the blood–brain barrier (BBB). The BBB is a highly selective semipermeable membrane, which separates the circulating blood from the brain and prevents the passage of the majority of the molecules in circulation so that central nervous system homeostasis is maintained [ 193 ]. Due to the highly selective nature of the BBB, only a small portion of therapeutic drugs can reach the brain. Therefore, high doses are required leading to adverse systemic effects. Nanoparticle-based therapeutic approaches in NDs mainly focus on targeted delivery and sustained local release of therapeutic agents into the affected area of the brain after crossing the BBB [ 194 , 195 ].
The aggregation of the amyloid-β (Aβ) peptide into amyloid plaques is the main pathological feature of AD, and current treatments include cholinesterase inhibitors (donepezil, rivastigmine, galantamine) and N -methyl- d -aspartate (NMDA) receptor antagonists (memantine) [ 196 ]. Reformulation of clinically used drugs with polymeric nanoparticles, non-polymeric quantum dots, and lipid-based nanoparticles enables their passage through BBB and reduces the side effects compared to free drug administration [ 197 , 198 , 199 , 200 , 201 , 202 ]. Concerning nano-delivery systems, there are also other attempts to cross the BBB and to reduce Aβ aggregates by using several neuroprotective compounds like metal chelators and various NMDA antagonists of anti-amyloids [ 203 , 204 ].
Parkinson’s disease (PD) is another type of neurodegenerative disease characterized by the selective degeneration of dopaminergic neurons and by the existence of α-synuclein as well as protein inclusions in neurons named Lewy bodies [ 205 ]. Dopamine replacement therapies are presently the most used strategy for PD treatment, since this class of drugs can help to improve the symptoms in motor neurons and is able to slow down the progression of the disease. However, the effect of these drugs on behavior and cognition is still in debate [ 206 ]. Recent research activities in nano-delivery focused on the development of therapeutic nanoparticles based on different strategies. Targeted delivery of dopamine using polymeric nanoparticles or liposomes is one of the nanoparticle-based therapeutic approaches in PD treatment [ 207 ]. Several studies use various drugs (Ropinirole, Bromocriptine, Mitoapocynin, Apomorphine) encapsulated with liposomes or polymeric nanoparticles in order to improve sustained release of drugs and to reduce undesired effects of conventional PD therapy [ 208 , 209 , 210 ]. Anti-inflammatory strategies are also developed by using polymeric nanoparticles or PEGylated liposomes to prevent neuronal cell death in PD [ 211 , 212 , 213 ]. As a neurotrophic strategy, PEGylated nanoparticles loaded with h-GDNF (glial cell-derived neurotrophic factor) improve locomotor activity and decrease the loss of dopaminergic neurons, which results in enhanced dopamine levels [ 214 , 215 ]. Moreover, polymer-based biodegradable nanoparticles have been engineered as cell therapeutics allowing stem cells to repair damaged nerves [ 216 ]. On the other hand, several groups proposed therapeutic nano-systems for the delivery of genetic material (DNA, RNA, and oligonucleotides) to inhibit faulty gene expression or synthesize therapeutic proteins in affected cells [ 217 ]. Although significant improvement in clinical symptoms is observed in advanced PD patients who underwent gene therapy, this approach is still a contradictive issue because of the heterogenic pathology of PD [ 218 ].
Despite many research articles on the development of novel therapeutic nanoparticles published for AD and PD, only few approaches have been reported for other neurodegenerative diseases, like amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS). ALS is a progressive neurodegenerative disease affecting motor neurons responsible for controlling voluntary muscle movements (chewing, walking, and talking) in the brain and spinal cord. Clinically, progressive muscle weakness results in death due to respiratory failure. To date, the only agent approved for treatment of ALS is Riluzole. Loading Riluzole in solid lipid nanoparticles promotes the delivery of the drug to the CNS (central nervous system), and pH-based controlled release is achieved with lower random biodistribution in organs such as the liver, spleen, heart, kidneys, and lung [ 219 , 220 ]. MS is characterized by the destruction of the protective coating (myelin sheath) on nerves of the central nervous system, which causes a faulty relay of instructions from the brain to the body. The conjugation of a glutamate receptor antagonist with a non-polymeric fullerene derivative nanoparticle is able to rescue the clinical progression of chronic MS in an in vivo model [ 221 ]. In 2015, Glatopa ® was approved by the FDA as the first generic drug, which is derived from the only therapeutic peptide, also approved by the FDA for the treatment of MS, glatiramer acetate (also known as the Copaxone ® ) [ 222 ]. Glatiramer acetate is a random copolymer and a synthetic peptide composed of l-lysine, l-alanine, l-glutamic acid, and l-tyrosine, which could suppress inflammatory responses by blocking MHC-II and changes the population of T-cell [ 102 ].
4.6. Ocular Diseases
Current ocular therapies taking advantage of nanomedicine can be enumerable as mydriatics or cycloplegics miotics, infection, and inflammatory, as well as diagnostic and surgical, adjuvants. However, several barriers, including the mucoaqueous tear layer, corneal epithelium, and the blood-retina barrier, make the eye impermeable for most therapeutic agents [ 223 ]. Targeted nano-delivery systems offer advantages in ocular disease therapy by lowering eye irritation and enhancing the bioavailability by providing a route of entry to the eye [ 224 ]. The most widely used nano-delivery systems are polymeric nanoparticles and liposomes developed for targeting the drugs to the right compartment of the eye, initially by increasing residence time on the tear film and enhancing corneal permeability [ 225 , 226 ]. Nano-formulation of the drug pranoprofen with the polymer PLGA (poly (lactic-co-glycolic acid) significantly enhanced its ophthalmic delivery and local anti-inflammatory and analgesic effects of the drug [ 227 ]. Similarly, chitosan-based polymeric nanoparticles encapsulating cefuroxime, diclofenac, or dexamethasone improved ocular bioavailability of the drugs [ 228 ]. These nanoparticles are able to interact with ocular surface while protecting the drug from metabolic degradation and extending pre-corneal residence [ 229 ]. Similarly, lipid-based nanoparticles loaded with brimonidine were used to treat an ophthalmic disease, glaucoma [ 230 , 231 , 232 ]. Immunologic graft rejection is a challenge in corneal transplantation. PLGA- or PEG-based polymeric formulations of dexamethasone and curcumin prevent the rejection of corneal graft due to the sustained release of the corticosteroids [ 233 , 234 ].
4.7. Pulmonary Diseases
Pulmonary lung diseases include asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, pulmonary tuberculosis, and idiopathic pulmonary fibrosis (IPF) [ 235 ]. These diseases are often fatal, and there is no effective treatment for completely restoring lung function. Conventional therapeutics are applied either systemically or locally to the lungs in the form of inhalers. Inhalation of free drugs, where the active molecules are in aerosol form, can lead to burst release and thus high lung toxicity. Moreover, the size of the aerosol limits the efficiency of the drug, as molecules >5 μm cannot pass the upper respiratory tract, particles between 1–5 μm mostly settle at the lower respiratory tract, and particles <1 μm stay suspended in air and exhaled. Nanoparticle-based delivery systems enable enhanced bioavailability, controlled release, and decreased dosage and application frequency. For the development of nanomedicine inhalation formulations, natural polymeric nanoparticles such as gelatin, chitosan, and alginate, as well as synthetic polymers like poloxamer, PLGA, and PEG, are widely used [ 236 , 237 ]. Moreover, polyamidoamine (PAMAM) dendrimers assembled with anti-asthma beclometasone dipropionate (BDP) were effectively used for pulmonary inhalation [ 238 ]. Besides, lipid-, polysaccharide-, or polymer-based nanoparticles and metallic or carbon-based nanoparticles were utilized for vaccine delivery or pulmonary immune hemostasis [ 239 ].
4.8. Regenerative Therapy
Regenerative therapy focuses on the design and application of biocompatible materials, which can enhance the repair and regeneration of tissues by making use of their natural cellular mechanisms. Stem cell-based therapy is one strategy for promoting tissue’s natural repair or regeneration mechanism.
Over the years, there has been increased interest in the development and direct administration of therapeutic nanoparticles to promote bone regeneration [ 240 ]. The most commonly used nano-delivery systems for bone regeneration are synthetic (PLA or PLGA) or natural polymers (collagen, gelatin, albumin, and chitosan). Besides the polymeric ones, various formulations of non-polymeric nanoparticles (silica-based, metallic) have also been used as nano-delivery systems for bone regeneration. For example, calcium phosphate-based non-polymeric nanoparticles are mostly used due to their similarities to human bone [ 241 , 242 , 243 ]. Delivering several growth factors is one of the nanoparticle-based therapeutic strategies based on the stimulation of osteoblasts for bone formation [ 244 , 245 , 246 , 247 ]. Moreover, nano-delivery of synthetic molecules is used as another therapeutic strategy in bone tissue, which could suppress the bone-resorbing cells, the osteoclasts. The bisphosphonate drugs promote osteoclasts apoptosis and are thus widely used for osteoporosis treatment. Several types of polymeric or non-polymeric metallic nanoparticles have been used to deliver bisphosphonate drugs [ 248 , 249 ].
Implantations of bone-graft substitutes stimulating mineralization are an alternative and effective approach for bone and dental tissue regeneration. EquivaBone ® is a nanotherapeutic that consists of hydroxyapatite, carboxymethyl cellulose, and demineralized bone matrix. EquivaBone ® is used as an osteoinductive bone graft substitute and was approved by FDA in 2009 [ 250 ].
Another strategy for the use of therapeutic nanoparticles in bone tissue is reducing inflammation, particularly in the case of large wounds. Synthetic or natural polymeric nanoparticles loaded with anti-inflammatory agents are delivered into the infected area, which could inhibit both inflammation and osteoblast resorption [ 251 , 252 ].
5. Limitations and Disadvantages of Therapeutic Nanoparticles
Utilization of nanoparticles provides promising results for the treatment of a large variety of diseases from cancer to glaucoma. Nanomedical approaches based on nanoparticle technologies, unfortunately, come with some limitations and disadvantages. Nanoparticle toxicity, eluding from the phagocytic system, refraining from the physiological barrier, and generating immune response are only some of the issues that should be taken into consideration carefully while using them in living organisms [ 253 ].
In vitro and in vivo studies showed that there is a relation between the size and toxicity of therapeutic nanoparticles. As nanoparticle sizes get smaller, dispersion to the nucleus steadily increases, which in return can cause intrinsic toxicity both at cellular and systemic level [ 254 , 255 ]. Another obstacle with smaller nanoparticles is their aggregation tendency. For instance, smaller size of micelles, dendrimers, and QDs are inclined to aggregation that results in poor biodistribution [ 256 , 257 , 258 , 259 ]. Although surface functionalization with PEG is a very effective method for reducing accumulation in non-target organs, these nanoparticles are often referred to as “stealth” nanoparticles because they can elude from the phagocytic system and may provoke cellular toxicity [ 260 ].
Therapeutic nanoparticles combined with drugs currently used in medical applications provide various features to the drug and increase the efficiency of the treatment. However, Manzoor et al. found that drug concentration and penetration into the tumor areas might be limited with nanoparticle-based drug delivery systems due to the heterogeneities of vascular permeability. In order to overcome the problem, they suggested a controlled delivery by using drug-loaded liposomes that are being triggered by local heat for drug release in vitro [ 261 ].
QDs are another type of contradictive nanoparticles for medical applications. As mentioned above, QDs have characteristic properties for fluorescent emission; thus, they are widely used for imaging applications. Beside their benefits during diagnosis of the disease, QDs have non-negligible disadvantages such as high intrinsic cytotoxicity. Research related to cadmium-based quantum dots showed that there is leakage of metal ions in QDs, resulting in high toxicity in hepatocyte cultures [ 262 , 263 , 264 ]. Shao et al. showed that effects of QDs could differ depending on the coating types as liposome or polymer. Another research indicated that quantum dot-lipid complex (QD-LC) has little effect on normal human hepatic cells and selectively kills cancer cells in a dose- and time-dependent manner in vivo. Furthermore, QD-LC nanoparticles trigger reactive oxygen species (ROS)-mediated apoptotic c-Jun N-terminal kinase (JNK) pathway in human liver cancer cells [ 265 ].
6. Conclusions
During the last decade, development of nanoparticle-based therapeutic agents has been extensively studied, and nano-delivery systems are the area of prime importance for specifically targeting the desired area in the treatment of many diseases.
Currently, the majority of nanoparticles used for the targeting delivery approach are made of polymers or lipids. Even though polymeric nanoparticles demonstrate great advantages in disease therapy, they also present disadvantages such as difficulties in scaling up, usage of organic solvents in their fabrication process, biocompatibility, cytotoxicity, and immunogenicity. On the other hand, lipid-based nanoparticles exhibit the ability to cross hard-to-reach sites, even without any surface functionalization, because of their similarity to cell membrane. Thus, lipid-based nano-delivery systems are considered as the next generation of therapeutics.
As of today, therapeutic nanoparticles are mostly developed for the treatment or prevention of only one disease. However, researchers started to combine various drug molecules as well as various types of nanoparticles, thereby, the future of therapeutic nanoparticles is guided to the direction of multi-therapeutic nanoparticles to be designed for the treatment of more than one disease.
Although nanoparticle-based delivery systems contribute significantly to the targeted therapy with improved efficiency, reduced side effects, and better bioavailability, we still know very little about the metabolism, clearance, and toxicity of nanoparticles. To date, most of the published studies demonstrate the encapsulation of clinical drugs with nanoparticles. However, the studies on other therapeutics, like genes, enzymes, or DNA/RNA, are still limited. The significance of published data is often approved with patents; however, a few of them can pass the trials and become commercially available in the market. This suggests that additional studies are required about the formulation, fabrication, and toxicity of nanoparticles. Moreover, cost of nanomedicine and manufacturing at larger scale is another important issue needing to be addressed. The lack of financial input or the poor cost–benefit balance prevents the progress of therapeutics from their inception to the market. Consequently, understanding the characteristics of nanoparticles and their interactions with their biological environment, like their targeting receptors or mechanisms of action in disease pathophysiology, will enable us to overcome limitations and to establish novel strategies for the treatment, prevention, and diagnosis in many diseases, particularly untreatable ones.
Nanomedicine will be the future of medicine, and nanoparticle-based therapeutics lies at the heart of it. However, a long ground should be gained before prosperity. Most importantly, long-term safety/toxicity of the nanoparticles should be investigated. Meanwhile, the discoveries on disease mechanisms and new drugs will lead to ways of placing more efficient and safer nanoparticle-based therapeutics in treatment regimens.
Author Contributions
A.A.Y.; writing—original draft preparation, S.C.; M.Z.; A.K.; review and editing, O.K.; supervision. All authors have read and agreed to the published version of the manuscript.
This work was supported by Sabanci University, Internal Grant for Research Project No: I.A.SN-17-01698.
Conflicts of Interest
The authors declare that there is no conflict of interest.
share this!
May 11, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Research explores ways to mitigate the environmental toxicity of ubiquitous silver nanoparticles
by Oregon State University
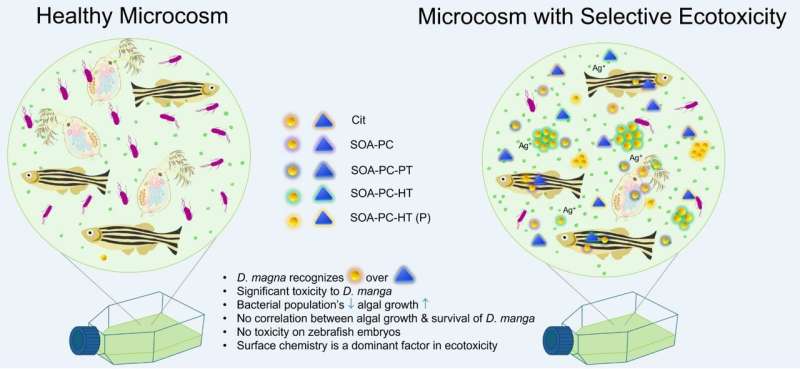
Silver has long been used to thwart the spread of illness and in recent years silver nanoparticles have been incorporated into products ranging from sanitizers, odor-resistant clothes and washing machines to makeup, food packaging and sports equipment.
Nanoparticles are tiny pieces of material ranging in size from one- to 100-billionths of a meter. In addition to their antimicrobial properties, silver nanoparticles are industrially important as catalysts and in electronics applications.
Despite their ubiquity, little is known about their environmental toxicity or how it might be mitigated.
Researchers at Oregon State University have taken a key step toward closing the knowledge gap with a study that indicates the particles' shape and surface chemistry play key roles in how they affect aquatic ecosystems.
The findings, published in Nanomaterials , are important because they suggest silver nanoparticles can be produced in formats that preserve their beneficial properties while limiting environmentally negative ones.
Scientists led by Marilyn Rampersad Mackiewicz and Stacey L. Harper assessed how spherical and triangular-shaped silver nanoparticles with five different surface chemistries affected their uptake and toxicity in a laboratory microcosm of bacteria, algae, Daphnia and embryonic zebrafish.
Daphnia are tiny crustaceans, and zebrafish are a small freshwater species that go from a cell to a swimming fish in about five days.
Zebrafish are particularly useful for studying the development and genetics of vertebrates, including the effects of environmental contaminants and pharmaceuticals on early embryonic development . They share a remarkable similarity to humans at the molecular, genetic and cellular levels; embryonic zebrafish are of special interest because in addition to developing quickly, they are transparent and can be easily maintained in small amounts of water.
The authors note that hundreds of tons of silver nanoparticles are produced every year for commercial uses, meaning it's inevitable some will end up in aquatic environments.
"Silver nanoparticles are not regulated by the Food and Drug Administration and not much is known about their toxicity except for the free silver ions that can result from surface oxidation of the nanoparticles," said Mackiewicz, assistant professor of chemistry. "Free silver ions are known to be toxic and in this paper we found a way to study the toxicity of silver nanoparticles and how they impact the environment irrespective of poisonous silver ions."
Mackiewicz, Harper and collaborators in the OSU colleges of Science, Engineering and Agricultural Sciences found silver nanoparticles negatively affect some species but not others.
"For example, there is a decrease in bacterial and Daphnia growth, and the size and shape of the particles can contribute to that, but the nanoparticles didn't affect zebrafish," she said. "And nanoparticles coated in lipids, organic compounds found in many natural oils and waxes, did not release significant amounts of silver ions—but they exhibited the greatest toxicity to Daphnia magna, the most sensitive species in the microcosm."
Overall, Mackiewicz said, the study showed that silver nanoparticles' shape and surface chemistry can be manipulated to achieve specific objectives necessary for better understanding and mitigating the risks associated with silver nanoparticles. A related study awaiting publication, she added, shows that small, spherical nanoparticles are more toxic than triangles or cubes.
Nanoparticles are the latest format, Mackiewicz notes, for an element that throughout history has been used to restrict the spread of human disease via incorporation into items used in everyday life. Its earliest recorded use for therapeutic purposes dates back 3,500 years.
During the Middle Ages, wealthy families used so many silver vessels, plates and other products that they developed bluish skin discolorations known as argyria, a condition believed to have led to the term "blue blood" as a description for members of the aristocracy.
Collaborating with Mackiewicz and Harper on the study were OSU researchers Bryan Harper and Arek Engstrom.
Provided by Oregon State University
Explore further
Feedback to editors

Blue Origin flies thrill seekers to space, including oldest astronaut
14 hours ago
Composition of gut microbiota could influence decision-making
May 18, 2024
Researchers realize multiphoton electron emission with non-classical light

Saturday Citations: Mediterranean diet racks up more points; persistent quantum coherence; vegan dogs

Physicists propose path to faster, more flexible robots

Scientists develop new geochemical 'fingerprint' to trace contaminants in fertilizer
May 17, 2024
Study reveals how a sugar-sensing protein acts as a 'machine' to switch plant growth—and oil production—on and off

Researchers develop world's smallest quantum light detector on a silicon chip


How heat waves are affecting Arctic phytoplankton

Horse remains show Pagan-Christian trade networks supplied horses from overseas for the last horse sacrifices in Europe
Relevant physicsforums posts, what was the original purpose of the chainsaw.
2 hours ago
DNA-maternity test - could you see other relationship than mother?
8 hours ago
And Now, here comes COVID-19 version BA.2, BA.4, BA.5,...
Is it usual for vaccine injection site to hurt again during infection.
May 16, 2024
A Brief Biography of Dr Virgina Apgar, creator of the baby APGAR test
May 12, 2024
Who chooses official designations for individual dolphins, such as FB15, F153, F286?
May 9, 2024
More from Biology and Medical
Related Stories
Self-embedding silver nanoparticles: Researchers find the 'silver lining' in cotton gin waste
Apr 10, 2024
Researchers develop first of its kind, simple test for identifying toxic silver ions
Jul 15, 2020

Video: How silver nanoparticles cut odors
Sep 20, 2018
Silver nanoparticles show promise in fighting antibiotic-resistant bacteria
Jan 31, 2023
Panning for silver in laundry wastewater
Dec 20, 2017
Improving safety assessment of nanoparticles
Jan 4, 2023
Recommended for you
Ion irradiation offers promise for 2D material probing

Using DNA origami, researchers create diamond lattice for future semiconductors of visible light
Nanobubble research to improve green hydrogen production

A golden layer unlocks sharper imaging and faster scanning with X-rays
Low-temperature pulse irradiation technique enables flexible optoelectronic devices
Nanocarriers loaded with DNA relieve back pain, repairs damaged disk in mice
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
REVIEW article
A review on nanoparticles: characteristics, synthesis, applications, and challenges.

- Department of Biology, College of Science, University of Hafr Al Batin, Hafr Al-Batin, Saudi Arabia
The significance of nanoparticles (NPs) in technological advancements is due to their adaptable characteristics and enhanced performance over their parent material. They are frequently synthesized by reducing metal ions into uncharged nanoparticles using hazardous reducing agents. However, there have been several initiatives in recent years to create green technology that uses natural resources instead of dangerous chemicals to produce nanoparticles. In green synthesis, biological methods are used for the synthesis of NPs because biological methods are eco-friendly, clean, safe, cost-effective, uncomplicated, and highly productive. Numerous biological organisms, such as bacteria, actinomycetes, fungi, algae, yeast, and plants, are used for the green synthesis of NPs. Additionally, this paper will discuss nanoparticles, including their types, traits, synthesis methods, applications, and prospects.
1. Introduction
Nanotechnology evolved as the achievement of science in the 21st century. The synthesis, management, and application of those materials with a size smaller than 100 nm fall under the interdisciplinary umbrella of this field. Nanoparticles have significant applications in different sectors such as the environment, agriculture, food, biotechnology, biomedical, medicines, etc. like; for treatment of waste water ( Zahra et al., 2020 ), environment monitoring ( Rassaei et al., 2011 ), as a functional food additives ( Chen et al., 2023 ), and as a antimicrobial agents ( Islam et al., 2022 ). Cutting-edge properties of NPs such as; nature, biocompatibility, anti-inflammatory and antibacterial activity, effective drug delivery, bioactivity, bioavailability, tumor targeting, and bio-absorption have led to a growth in the biotechnological, and applied microbiological applications of NPs.
A particle of matter with a diameter of one to one hundred nanometers (nm) is commonly referred to as a nanoparticle or ultrafine particle. Nanoparticles frequently exhibit distinctive size-dependent features, mostly due to their tiny size and colossal surface area. The periodic boundary conditions of the crystalline particle are destroyed when the size of a particle approaches the nano-scale with the characteristic length scale close to or smaller than the de Broglie wavelength or the wavelength of light ( Guo et al., 2013 ). Because of this, many of the physical characteristics of nanoparticles differ significantly from those of bulk materials, leading to a wide range of their novel uses ( Hasan, 2015 ).
2. Emergence of nanotechnology
Nanotechnology emerged in the 1980s due to the convergence of experimental advances such as the invention of the scanning tunneling microscope in 1981 and the discovery of fullerenes in 1985 ( Bayda et al., 2019 ), with the elucidation. The popularization of a conceptual framework for nanotechnology goals began with the publication of the book Engines of Creation in 1986 ( Bayda et al., 2019 ).
2.1. Early stage of NPs
Carbon nanotubes have been discovered in pottery from Keeladi, India, dating from around 600–300 BC ( Bayda et al., 2019 ; Kokarneswaran et al., 2020 ). Cementite nanowires have been discovered in Damascus steel, a material that dates back to around 900 AD; nevertheless, its origin and creation method are unclear ( Kokarneswaran et al., 2020 ). However, it is unknown how they developed or whether the material containing them was used on purpose.
2.2. Discovery of C, Ag, Zn, Cu, and Au nanoparticles
Carbon NPs were found in 1991, and Iijima and Ichihashi announced the single-wall carbon nanotube synthesis with a diameter of 1 nanometer in 1993 ( Chen et al., 2021 ). Carbon nanotubes (CNTs), also known as Bucky tubes, are a kind of nanomaterial made up of a two-dimensional hexagonal lattice of carbon atoms. They are bent one way and joined to produce a hollow cylindrical cylinder. Carbon nanotubes are carbon allotropes that fall between Fullerene (0 dimensional) and Grapheme (2 dimensional) ( Chen et al., 2021 ).
In addition, M. C. Lea reported that the synthesis of citrate-stabilized silver colloid almost 120 years ago ( Nowack et al., 2011 ). This process produces particles with an average diameter of 7 to 9 nm. Nanoscale size and citrate stabilization are analogous to recent findings on nanosilver production employing silver nitrate and citrate ( Majeed Khan et al., 2011 ). The use of proteins to stabilize nanosilver has also been documented as early as 1902 ( Nowack et al., 2011 ; Beyene et al., 2017 ). Since 1897, a nanosilver known as “Collargol” has been made commercially and used for medicinal purposes ( Nowack et al., 2011 ). Collargol, a type of silver nanoparticle, has a particle size of about 10 nanometers (nm). This was determined as early as 1907, and it was found that the diameter of Collargol falls within the nanoscale range. In 1953, Moudry developed a different type of silver nanoparticle called gelatin-stabilized silver nanoparticles, with a diameter ranging from 2–20 nm. These nanoparticles were produced using another method than Collargol. The necessity of nanoscale silver was recognized by the creators of nanosilver formulations decades ago, as seen by the following remark from a patent: “for optimal efficiency, the silver must be disseminated as particles of colloidal size less than 25 nm in crystallite size”( Nowack et al., 2011 ).
Gold NPs (AuNPs) have a long history in chemistry, going back to the Roman era when they were used to decorate glassware by staining them. With the work of Michael Faraday, who may have been the first to notice that colloidal gold solutions have characteristics different from bulk gold, the contemporary age of AuNP synthesis began more than 170 years ago. Michael Faraday investigated the making and factors of colloidal suspensions of “Ruby” gold in 1857. They are among the magnetic nanoparticles due to their distinctive optical and electrical characteristics. Under specific illumination circumstances, Faraday showed how gold nanoparticles might create solutions of various colors ( Bayda et al., 2019 ; Giljohann et al., 2020 ).
3. Classification of NPs
Nanoparticles (NPs) are categorized into the following classes based on their shape, size, and chemical characteristics
3.1. Carbon-based NPs
Fullerenes and carbon nanotubes (CNTs) are the two essential sub-categories of carbon-based NPs. NPs of globular hollow cages, like allotropic forms of carbon, are found in fullerenes. Due to their electrical conductivity, high strength, structure, electron affinity, and adaptability, they have sparked significant economic interest. These materials have organized pentagonal and hexagonal carbon units, each of which is sp2 hybridized. While CNTs are elongated and form 1–2 nm diameter tubular structures. These fundamentally resemble graphite sheets rolling on top of one another. Accordingly, they are referred to as single-walled (SWNTs), double-walled (DWNTs), or multi-walled carbon nanotubes (MWNTs) depending on how many walls are present in the rolled sheets ( Elliott et al., 2013 ; Astefanei et al., 2015 ).
3.2. Metal NPs
Metal NPs are purely made of metals. These NPs have distinctive electrical properties due to well-known localized surface Plasmon resonance (LSPR) features. Cu, Ag, and Au nanoparticles exhibit a broad absorption band in the visible region of the solar electromagnetic spectrum. Metal NPs are used in several scientific fields because of their enhanced features like facet, size, and shape-controlled synthesis of metal NPs ( Khan et al., 2019 ).
3.3. Ceramics NPs
Ceramic NPs are tiny particles made up of inorganic, non-metallic materials that are heat-treated and cooled in a specific way to give particular properties. They can come in various shapes, including amorphous, polycrystalline, dense, porous, and hollow, and they are known for heat resistance and durable properties. Ceramic NPs are used in various applications, including coating, catalysts, and batteries ( Sigmund et al., 2006 ).
3.4. Lipid-based NPs
These NPs are helpful in several biological applications because they include lipid moieties. Lipid NPs typically have a diameter of 10–1,000 nm and are spherical. Lipid NPs, i.e., polymeric NPs, have a solid lipid core and a matrix consisting of soluble lipophilic molecules ( Khan et al., 2019 ).
3.5. Semiconductor NPs
Semiconductor NPs have qualities similar to metals and non-metals. That is why Semiconductor NPs have unique physical and chemical properties that make them useful for various applications. For example, semiconductor NPs can absorb and emit light and can be used to make more efficient solar cells or brighter light-emitting diodes (LEDs). They can make smaller and faster electronic devices, such as transistors, and can be used in bio imaging and cancer therapy ( Biju et al., 2008 ).
3.6. Polymeric NPs
Polymeric NPs with a size between 1 and 1,000 nm can have active substances surface-adsorbed onto the polymeric core or entrapped inside the polymeric body. These NPs are often organic, and the term polymer nanoparticle (PNP) is commonly used in the literature to refer to them. They resemble Nano spheres or Nano capsules for the most part ( Khan et al., 2019 ; Zielińska et al., 2020 ).
4. Types of different metal-based NPs
Metal NPs are purely made of metal precursors. Due to well-known localized surface plasmon resonance (LSPR) characteristics, these NPs possess unique optoelectrical properties. NPs of the alkali and noble metals, i.e., Cu, Ag, and Au, have a broad absorption band in the visible zone of the solar electromagnetic spectrum. The facet, size, and shape-controlled synthesis of metal NPs are essential in present-day cutting-edge materials ( Dreaden et al., 2012 ; Khan et al., 2019 ).
4.1. Silver nanoparticles (AgNPs)
AgNPs are particles with a size range of 1–100 nanometers made of silver. They have unique physical and chemical properties due to their small size, high surface area-to-volume ratio, and ability to absorb and scatter light in the visible and near-infrared range. Because of their relatively small size and high surface-to-volume ratios, which cause chemical and physical differences in their properties compared to their bulk counterparts, silver nanoparticles may exhibit additional antimicrobial capabilities not exerted by ionic silver ( Shenashen et al., 2014 ).
Besides, AgNPs can be created in various sizes and forms depending on the manufacturing process, the most common of which is chemical reduction. The AgNPs were created by chemically reducing a 12 mM AgNO3 aqueous solution. The reaction was carried out in an argon environment using 70 mL of this solution containing PVP (keeping the molar ratio of the repeating unit of PVP and Ag equal to 34) and 21 mL of Aloe Vera. The mixture was agitated in ultrasonic for 45 min at ambient temperature, then heated 2°C/min to 80°C and left for 2 h to generate a transparent solution with tiny suspended particles that must be removed by simple filtering ( Shenashen et al., 2014 ; Gloria et al., 2017 ).
4.2. Zinc nanoparticles (ZnONPs)
Zinc nanoparticles (ZnONPs) are particles with a size range of 1–100 nm made of zinc. Zinc oxide (ZnO) NPs are a wide band gap semiconductor with a room temperature energy gap of 3.37 eV. Its catalytic, electrical, optoelectronic, and photochemical capabilities have made it widely worthwhile ( Kumar S.S. et al., 2013 ). ZnO nanostructures are ideal for catalytic reaction processes ( Chen and Tang, 2007 ). Laser ablation, hydrothermal methods, electrochemical depositions, sol-gel method, chemical vapor deposition, thermal decomposition, combustion methods, ultrasound, microwave-assisted combustion method, two-step mechanochemical-thermal synthesis, anodization, co-precipitation, electrophoretic deposition, and precipitation processes are some methods for producing ZnO nanoparticles ( Madathil et al., 2007 ; Moghaddam et al., 2009 ; Ghorbani et al., 2015 ).
4.3. Copper nanoparticles (CuNPs)
Copper nanoparticles (CuNPs) comprise a size range of 1–100 nm of copper-based particles ( Khan et al., 2019 ). Cu and Au metal fluorescence have long been known to exist. For excitation at 488 nm, a fluorescence peak centering on the metals’ interband absorption edge has been noted. Additionally, it was noted that the fluorescence peaked at the same energy at two distinct excitation wavelengths (457.9–514.5 and 300–400 nm), and the high-energy tail somewhat grows with increased photon energy pumping. A unique, physical, top-down EEW approach has been used to create Cu nanoparticles. The EEW method involves sending a current of *1,010 A/m2 (1,010 A/m2) across a thin Cu wire, which explodes on a Cu plate for a duration of 10–6 s ( Siwach and Sen, 2008 ).
4.4. Gold nanoparticles (AuNPs)
Gold nanoparticles(AuNPs) are nanometers made of gold. They have unique physical and chemical properties and can absorb and scatter light in the visible and near-infrared range ( Rad et al., 2011 ; Compostella et al., 2017 ).
Scientists around the turn of the 20th century discovered anisotropic AuNPs. Zsigmond ( Li et al., 2014 ) said that gold particles “are not always spherical when their size is 40 nm or lower” in his book, released in 1909. Additionally, he found anisotropic gold particles of various colors. Zsigmondy won the Nobel Prize in 1925 for “his demonstration of the heterogeneous character of colloidal solutions and the methods he utilized” and for developing the ultramicroscope, which allowed him to see the forms of Au particles. He noticed that gold frequently crystallized into a six-sided leaf shape ( Li et al., 2014 ).
AuNPs are the topic of extensive investigation due to their optical, electrical, and molecular-recognition capabilities, with numerous prospective or promised uses in a wide range of fields, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine ( Rad et al., 2011 ).
4.5. Aluminum nanoparticles (AlNPs)
Aluminum nanoparticles (AlNPs) are nanoparticles made of aluminum. Aluminum nanoparticles’ strong reactivity makes them promising for application in high-energy compositions, hydrogen generation in water processes, and the synthesis of alumina 2D and 3D structures ( Lerner et al., 2016 ).
4.6. Iron nanoparticles (FeNPs)
Iron nanoparticles(FeNPs) are particles with a size range of 1−100 nanometers ( Khan et al., 2019 ) made of iron. FeNPs have several potential applications, including their use as catalysts, drug delivery systems, sensors, and energy storage and conversion. They have also been investigated for use in photovoltaic and solar cells and water purification and environmental remediation. FeNPs can also be used in magnetic resonance imaging (MRI) as contrast agents to improve the visibility of tissues and organs. They can also be used in magnetic recording media, such as hard disk drives ( Zhuang and Gentry, 2011 ; Jamkhande et al., 2019 ).
As with any NPs, there are potential health and safety concerns associated with using FeNPs, e.g., FeNPs are used to deliver drugs to specific locations within the body, such as cancer cells and used in MRI, and used to remove contaminants from water ( Farrell et al., 2003 ; Zhuang and Gentry, 2011 ). Tables 1 , 2 show the characteristics of metal-based nanoparticles and the techniques to study their characteristics, respectively.
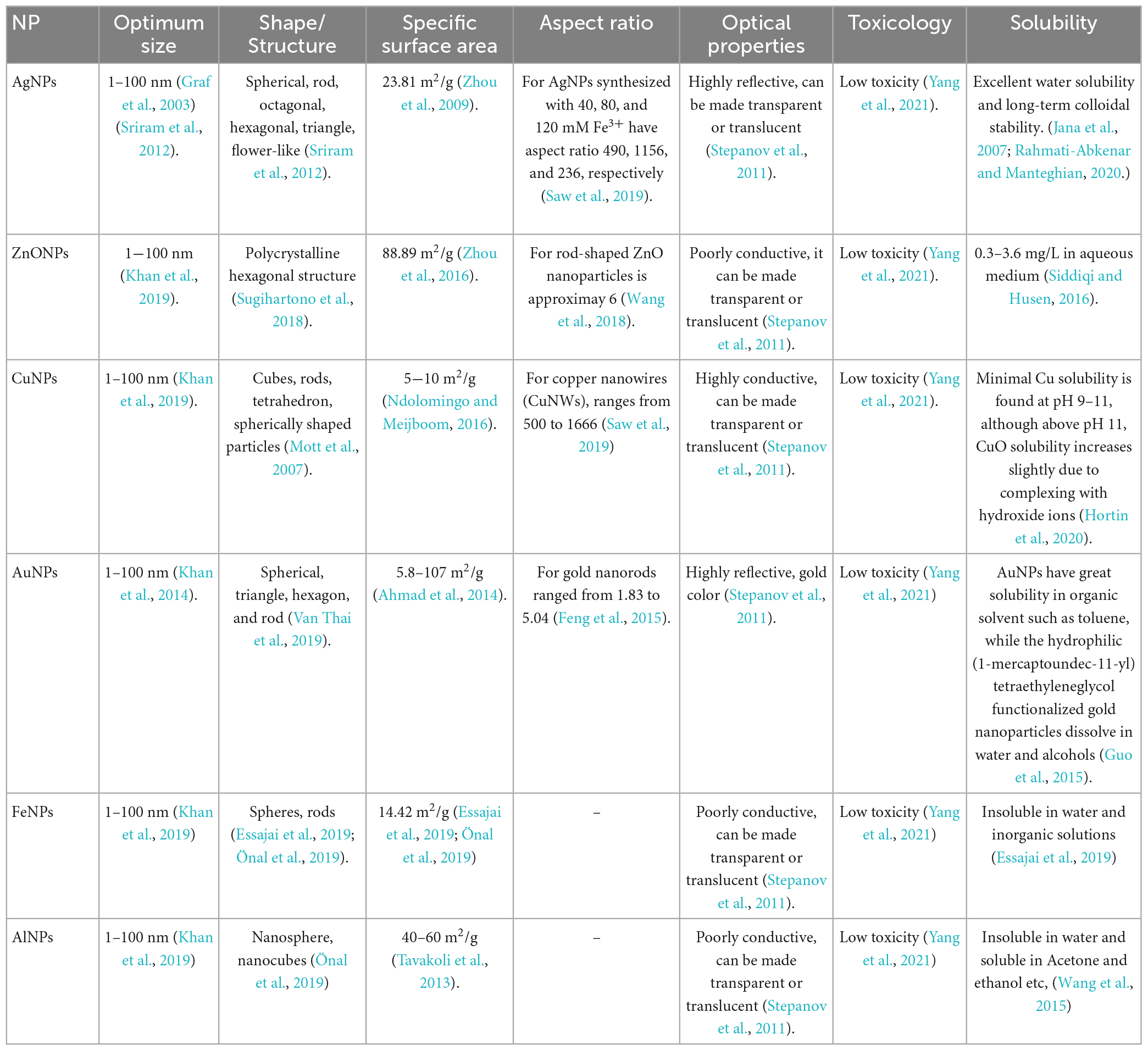
Table 1. Characteristics of metal based nanoparticles.
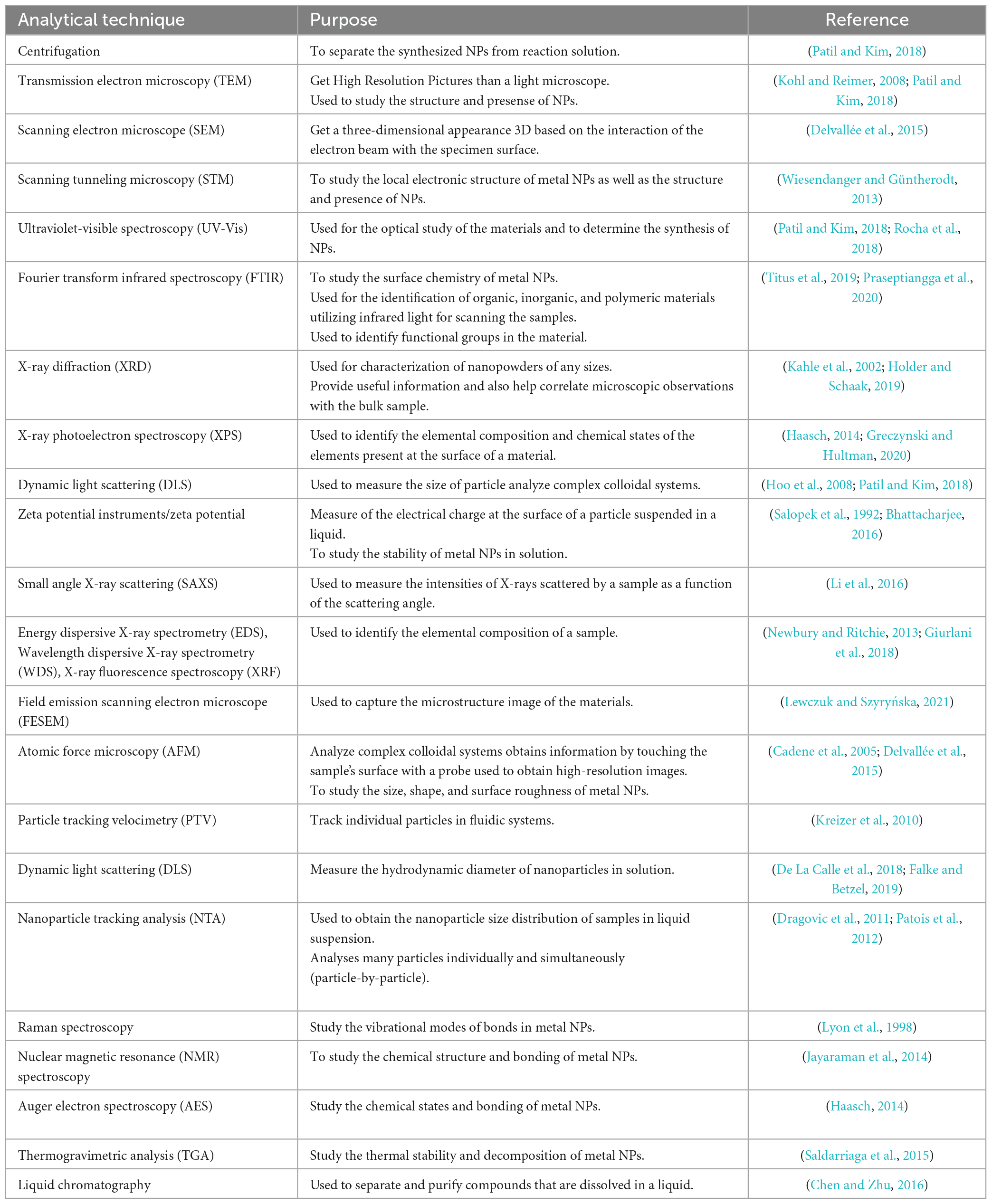
Table 2. Different analytical techniques and their purposes in studying nanoparticles.
5. Approaches for the synthesis of metal NPs
There are mainly three types of approaches for the synthesis of NPs: the physical, chemical, and biological approaches. The physical approach is also called the top-down approach, while chemical and biological approaches are collectively called the bottom-up approach. The biological approach is also named green systems of NPs. All these approaches are further sub-categorized into various types based upon their method adopted. Figure 1 illustrates each approach’s reported methods for synthesizing NPs.
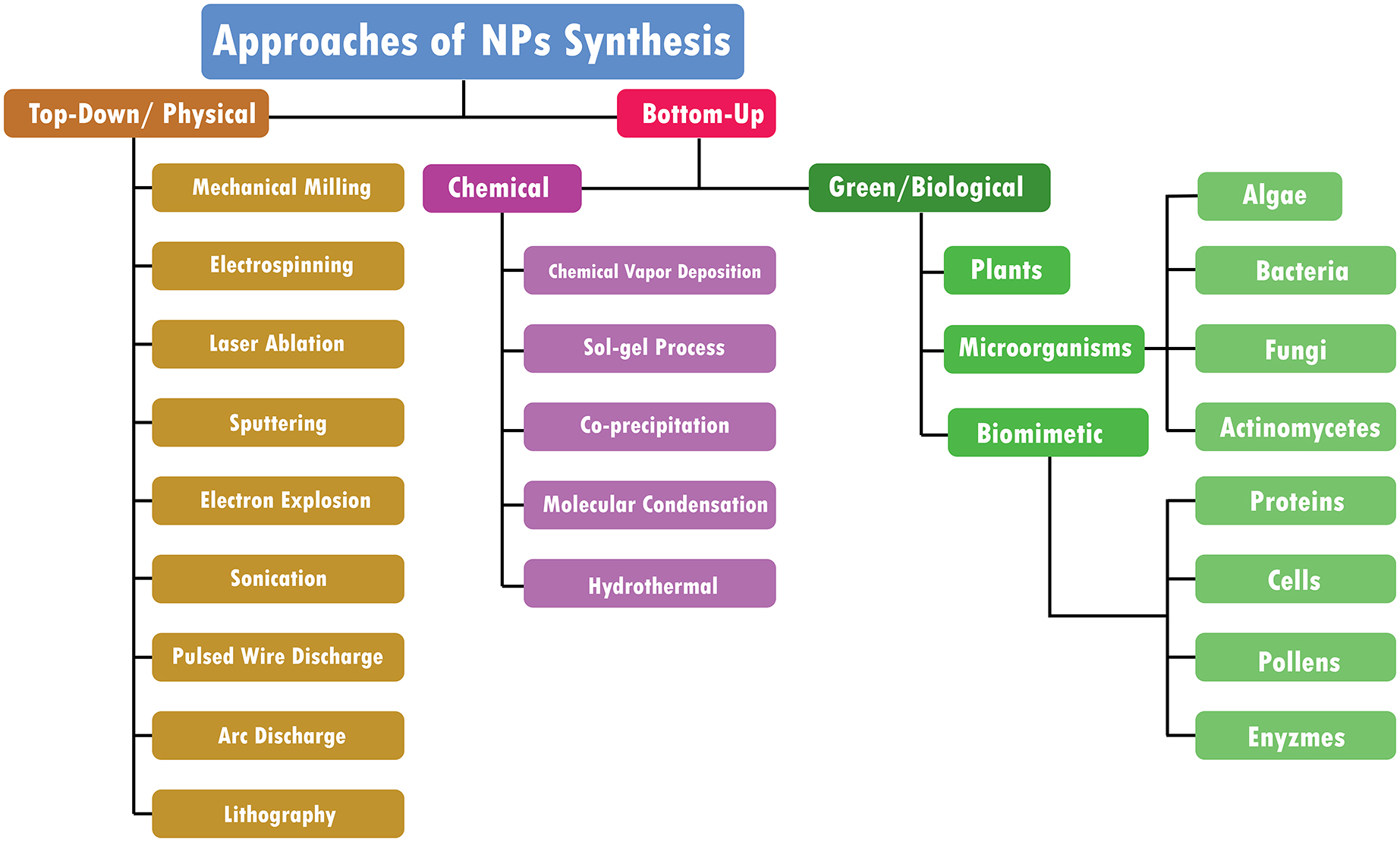
Figure 1. Approaches of NPs synthesis.
5.1. Top down/physical approach
Bulk materials are fragmented in top-down methods to create nano-structured materials ( Figure 2 ). They are additionally known as physical approaches ( Baig et al., 2021 ). The following techniques can achieve a top-down approach
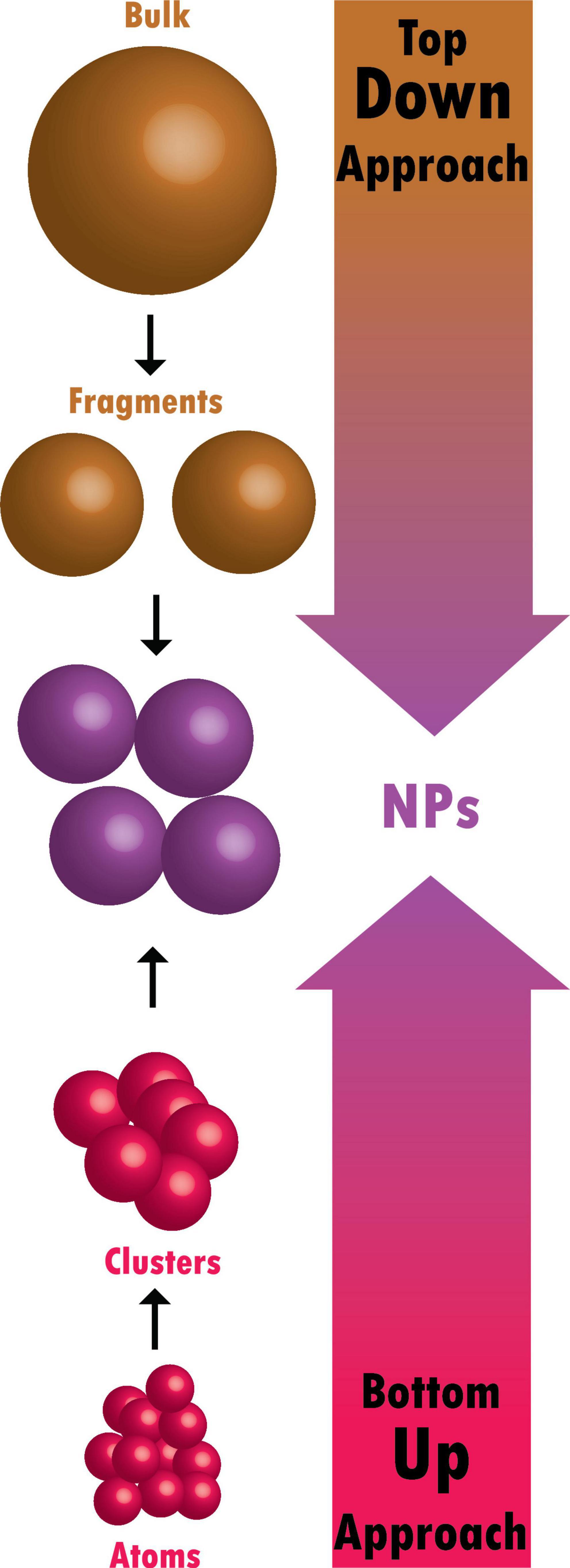
Figure 2. Difference between top-down and bottom-up approaches.
5.1.1. Mechanical milling
The mechanical milling process uses balls inside containers and may be carried out in various mills, typically planetary and shaker mills, which is an impact process with high energy ( Gorrasi and Sorrentino, 2015 ). Mechanical milling is a practical approach for creating materials at the nanoscale from bulk materials. Aluminum alloys that have been strengthened by oxide and carbide, spray coatings that are resistant to wear, nanoalloys based on aluminum, nickel, magnesium, and copper, and a variety of other nanocomposite materials may all be created mechanically. A unique class of nanoparticles known as ball-milled carbon nanomaterials has the potential to meet the needs for energy storage, energy conversion, and environmental remediation ( Yadav et al., 2012 ; Lyu et al., 2017 ).
5.1.2. Electrospinning
Typically, it is used to create nanofibers from various materials, most often polymers ( Ostermann et al., 2011 ). A technique for creating fibers called electrospinning draws charged threads from polymer melts or solutions up to fiber sizes of a few hundred nanometers ( Chronakis, 2010 ). Coaxial electrospinning was a significant advancement in the field of electrospinning. The spinneret in coaxial electrospinning is made up of two coaxial capillaries. Core-shell nanoarchitectures may be created in these capillaries using two viscous liquids, a viscous liquid as the shell and a non-viscous liquid as the core ( Du et al., 2012 ). Core-shell and hollow polymer, inorganic, organic, and hybrid materials have all been developed using this technique ( Kumar R. et al., 2013 ).
5.1.3. Laser ablation
A microfeature can be made by employing a laser beam to vaporize a single material ( Tran and Wen, 2014 ). Laser ablation synthesis produces nanoparticles by striking the target material with an intense laser beam. Due to the high intensity of the laser irradiation used in the laser ablation process, the source material or precursor vaporizes, causing the production of nanoparticles ( Amendola and Meneghetti, 2009 ). Laser ablation is an environmentally friendly for producing noble metal nanoparticles ( Baig et al., 2021 ). This method may be used to create a wide variety of nanomaterials, including metal nanoparticles, carbon nanomaterials, oxide composites, and ceramics ( Su and Chang, 2018 ; Baig et al., 2021 ).
5.1.4. Sputtering
Microparticles of a solid material are expelled off its surface during the phenomenon known as sputtering, which occurs when the solid substance is assaulted by intense plasma or gas particles ( Behrisch, 1981 ). According to the incident gaseous ion energy, energetic gaseous ions used in the sputtering deposition process physically expel tiny atom clusters off the target surface ( Muñoz-García et al., 2009 ). The sputtering method is intriguing because it is more affordable than electron-beam lithography, and the composition of the sputtered nanomaterials is similar to the target material with fewer contaminants ( Baig et al., 2021 ).
5.1.5. Electron explosion
In this technique, a thin metal wire is subjected to a high current pulse that causes an explosion, evaporation, and ionization. The metal becomes vaporized and ionized, expands, and cools by reacting with the nearby gas or liquid medium. The condensed vapor finally forms the nanoparticles ( Joh et al., 2013 ). Electron explosion method because it produces plasma from the electrical explosion of a metallic wire, which may produce nanoparticles from a Pt solution without using a reducing agent ( Joh et al., 2013 ).
5.1.6. Sonication
The most crucial step in the creation of nanofluids is sonication. After the mixture has been magnetically stirred in a magnetic stirrer, sonication is performed in an ultrasonication path, ultrasonic vibrator, and mechanical homogenizer. Sonicators have become the industry standard for Probe sonication and are noticeably more powerful and effective when compared to ultrasonic cleaner baths for nanoparticle applications. Probe sonication is highly effective for processing nanomaterials (carbon nanotubes, graphene, inks, metal oxides, etc.) ( Zheng et al., 2010 ).
5.1.7. Pulsed wire discharge method
This is the most used method for creating metal nanoparticles. A pulsating current causes a metal wire to evaporate, producing a vapor that is subsequently cooled by an ambient gas to form nanoparticles. This plan may quickly produce large amounts of energy ( Patil et al., 2021 ).
5.1.8. Arc discharge method
Two graphite rods are adjusted in a chamber with a constant helium pressure during the Arc Discharge procedure. It is crucial to fill the chamber with helium because oxygen or moisture prevents the synthesis of fullerenes. Arc discharge between the ends of the graphite rods drives the vaporization of carbon rods. Achieving new types of nanoparticles depends significantly on the circumstances in which arc discharge occurs. The creation of several nanostructured materials may be accomplished with this technique ( Berkmans et al., 2014 ). It is well-recognized for creating carbon-based materials such as fullerenes, carbon nanohorns (CNHs), carbon nanotubes ( Shi et al., 2000 ), few-layer graphene, and amorphous spherical carbon nanoparticles ( Kumar R. et al., 2013 ).
5.1.9. Lithography
Lithography typically uses a concentrated beam of light or electrons to create nanoparticles, a helpful technique ( Pimpin and Srituravanich, 2012 ). Masked and maskless lithography are the two primary categories of lithography. Without a mask, arbitrary nano-pattern printing is accomplished in maskless lithography. Additionally, it is affordable and easy to apply ( Brady et al., 2019 ).
5.2. Bottom-up approach
Tiny atoms and molecules are combined in bottom-up methods to create nano-structured particles ( Figure 2 ; Baig et al., 2021 ). These include chemical and biological approaches:
5.2.1. Chemical vapor deposition (CVD)
Through a chemical process involving vapor-phase precursors, a thin coating is created on the substrate surface during CVD ( Dikusar et al., 2009 ). Precursors are deemed appropriate for CVD if they exhibit sufficient volatility, high chemical purity, strong evaporation stability, cheap cost, a non-hazardous nature, and long shelf life. Additionally, its breakdown should not leave behind any contaminants. Vapor phase epitaxy, metal-organic CVD, atomic layer epitaxy, and plasma-enhanced CVD are only a few CVD variations. This method’s benefits include producing very pure nanoparticles that are stiff, homogeneous, and strong ( Ago, 2015 ). CVD is an excellent approach to creating high-quality nanomaterials ( Machac et al., 2020 ). It is also well-known for creating two-dimensional nanoparticles ( Baig et al., 2021 ).
5.2.2. Sol-gel process
A wet-chemical approach, called the sol-gel method, is widely utilized to create nanomaterials ( Das and Srivasatava, 2016 ; Baig et al., 2021 ). Metal alkoxides or metal precursors in solution are condensed, hydrolyzed, and thermally decomposed. The result is a stable solution or sol. The gel gains greater viscosity as a result of hydrolysis or condensation. The particle size may be seen by adjusting the precursor concentration, temperature, and pH levels. It may take a few days for the solvent to be removed, for Ostwald ripening to occur, and for the phase to change during the mature stage, which is necessary to enable the growth of solid mass. To create nanoparticles, the unstable chemical ingredients are separated. The generated material is environmentally friendly and has many additional benefits thanks to the sol-gel technique ( Patil et al., 2021 ). The uniform quality of the material generated, the low processing temperature, and the method’s ease in producing composites and complicated nanostructures are just a few of the sol-gel technique’s many advantages ( Parashar et al., 2020 ).
5.2.3. Co-precipitation
It is a solvent displacement technique and is a wet chemical procedure. Ethanol, acetone, hexane, and non-solvent polymers are examples of solvents. Polymer phases can be either synthetic or natural. By mixing the polymer solution, fast diffusion of the polymer-solvent into the non-solvent phase of the polymer results. Interfacial stress at two phases results in the formation of nanoparticles ( Das and Srivasatava, 2016 ). This method’s natural ability to produce high quantities of water-soluble nanoparticles through a straightforward process is one of its key benefits. This process is used to create many commercial iron oxide NP-based MRI contrast agents, including Feridex, Reservist, and Combidex ( Baig et al., 2021 ; Patil et al., 2021 ).
5.2.4. Inert gas condensation/molecular condensation
Metal NPs are produced using this method in large quantities. Making fine NPs using the inactive gas compression approach has been widespread, which creates NPs by causing a metallic source to disappear in an inert gas. At an attainable temperature, metals evaporate at a tolerable pace. Copper metal nanoparticles are created by vaporizing copper metal inside a container containing argon, helium, or neon. The atom quickly loses its energy by cooling the vaporized atom with an inert gas after it boils out. Liquid nitrogen is used to cool the gases, forming nanoparticles in the range of 2–100 nm ( Pérez-Tijerina et al., 2008 ; Patil et al., 2021 ).
5.2.5. Hydrothermal
In this method, for the production of nanoparticles, hydrothermal synthesis uses a wide temperature range from ambient temperature to extremely high temperatures. Comparing this strategy to physical and biological ones offers several benefits. At higher temperature ranges, the nanomaterials produced by hydrothermal synthesis could become unstable ( Banerjee et al., 2008 ; Patil et al., 2021 ).
5.2.6. Green/biological synthesis
The synthesis of diverse metal nanoparticles utilizing bioactive agents, including plant materials, microbes, and various biowastes like vegetable waste, fruit peel waste, eggshell, agricultural waste, algae, and so on, is known as “green” or “biological” nanoparticle synthesis ( Kumari et al., 2021 ). Developing dependable, sustainable green synthesis technologies is necessary to prevent the formation of undesirable or dangerous byproducts ( Figure 3 ). The green synthesis of nanoparticles also has several advantages, including being straightforward, affordable, producing NPs with high stability, requiring little time, producing non-toxic byproducts, and being readily scaled up for large-scale synthesis ( Malhotra and Alghuthaymi, 2022 ).
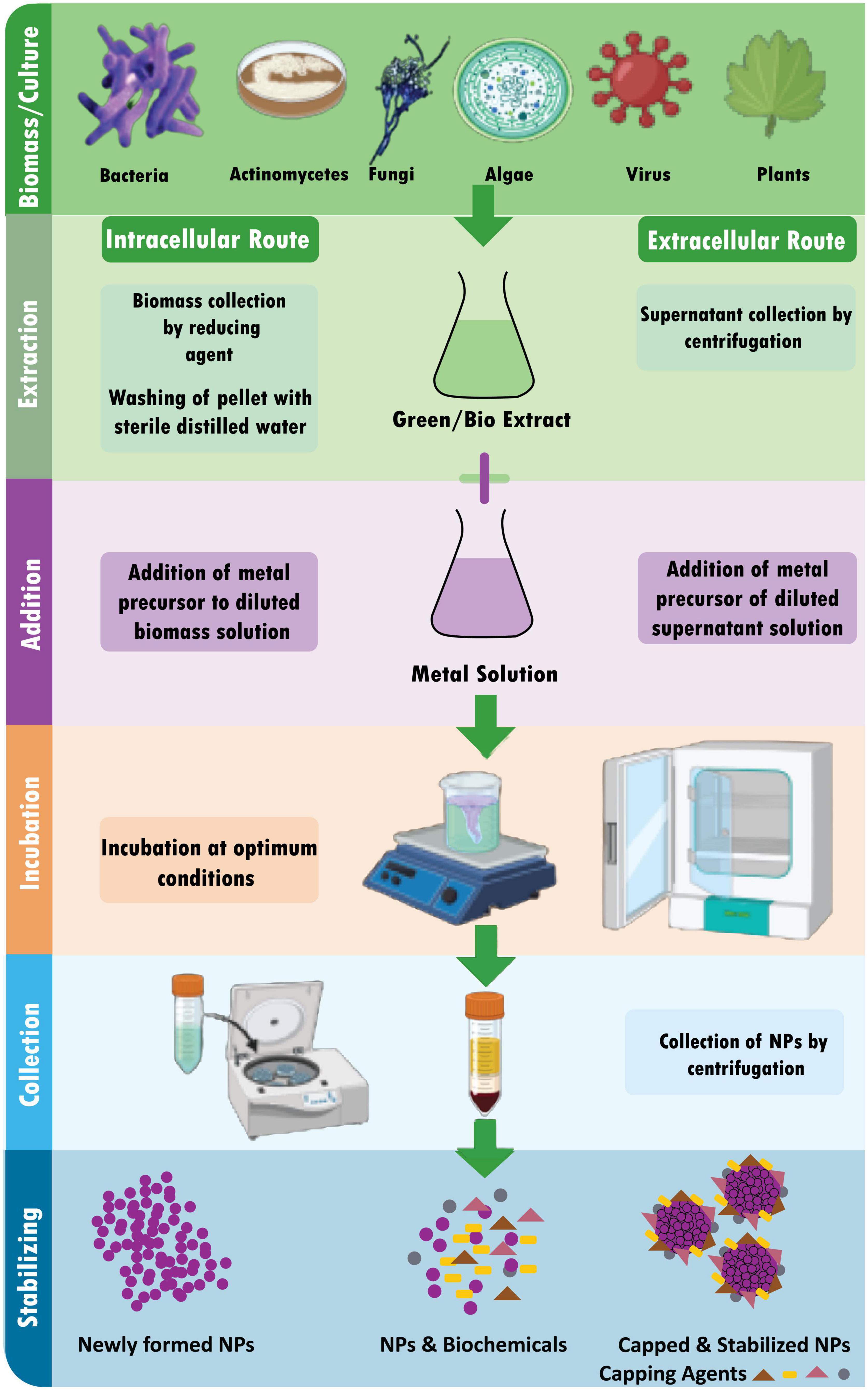
Figure 3. Schematic diagram for biosynthesis of NPs.
5.2.6.1. Biological synthesis using microorganisms
Microbes use metal capture, enzymatic reduction, and capping to create nanoparticles. Before being converted to nanoparticles by enzymes, metal ions are initially trapped on the surface or interior of microbial cells ( Ghosh et al., 2021 ). Use of microorganisms (especially marine microbes) for synthesis of metalic NPs is environmental friendly, fast and economical ( Patil and Kim, 2018 ). Several microorganisms are used in the synthesis of metal NPs, including:
Biosynthesis of NPs by bacteria: A possible biofactory for producing gold, silver, and cadmium sulfide nanoparticles is thought to be bacterial cells. It is known that bacteria may produce inorganic compounds either inside or outside of their cells ( Hulkoti and Taranath, 2014 ). Desulforibrio caledoiensis ( Qi et al., 2013 ), Enterococcu s sp. ( Rajeshkumar et al., 2014 ), Escherichia coli VM1 ( Maharani et al., 2016 ), and Ochrobactrum anhtropi ( Thomas et al., 2014 ) based metal NPs are reported previously for their potential photocatalytic properties ( Qi et al., 2013 ), antimicrobial activity ( Rajeshkumar et al., 2014 ), and anticancer activity ( Maharani et al., 2016 ).
Extracellular synthesis of NPs by bacteria: The microorganisms’ extracellular reductase enzymes shrink the silver ions to the nanoscale range. According to protein analysis of microorganisms, the NADH-dependent reductase enzyme carries out the bio-reduction of silver ions to AgNPs. The electrons for the reductase enzyme come from NADH, which is subsequently converted to NAD+. The enzyme is also oxidized simultaneously when silver ions are reduced to nanosilver. It has been noted that bio-reduction can occasionally be caused by nitrate-dependent reductase. The decline occurs within a few minutes in the quick extracellular creation of nanoparticles ( Mathew et al., 2010 ). At pH 7, the bacterium R. capsulata produced gold nanoparticles with sizes ranging from 10−20 nm. Numerous nanoplates and spherical gold nanoparticles were produced when the pH was changed to four ( Sriram et al., 2012 ). By adjusting the pH, the gold nanoparticles’ form may be changed. Gold nanoparticle shape was controlled by regulating the proton content at various pH levels. The bacteria R. capsulata ’s release cofactor NADH and NADH-dependent enzymes may cause the bioreduction of Au (3+) to Au (0) and the generation of gold nanoparticles. By using NADH-dependent reductase as an electron carrier, it is possible to start the reduction of gold ions ( Sriram et al., 2012 ).
Intracellular synthesis of NPs by bacteria: Three processes are involved in the intracellular creation of NPs: trapping, bioreduction, and capping. The cell walls of microorganisms and ions charge contribute significantly to creating NPs in the intracellular route. This entails specific ion transit in the presence of enzymes, coenzymes, and other molecules in the microbial cell. Microbes have a range of polysaccharides and proteins in their cell walls, which function as active sites for the binding of metal ions ( Slavin et al., 2017 ). Not all bacteria can produce metal and metal oxide nanoparticles. The only ions that pose a significant hazard to microorganisms are heavy metal ions, which, in response to a threat, cause the germs to react by grabbing or trapping the ions on the cell wall via electrostatic interactions. This occurs because a metal ion is drawn to the cell wall’s carboxylate groups, including cysteine and polypeptides, and certain enzymes with a negative charge ( Zhang et al., 2011 ).
Additionally, the electron transfers from NADH via NADH-dependent educates, which serves as an electron carrier and is located inside the plasma membrane, causing the trapped ions to be reduced into the elemental atom. The nuclei eventually develop into NPs and build up in the cytoplasm or the pre-plasmic space. On the other hand, the stability of NPs is provided by proteins, peptides, and amino acids found inside cells, including cysteine, tyrosine, and tryptophan ( Mohd Yusof et al., 2019 ).
Biosynthesis of NPs by fungi: Because monodisperse nanoparticles with distinct dimensions, various chemical compositions, and sizes may be produced, the biosynthesis of nanoparticles utilizing fungus is frequently employed. Due to the existence of several enzymes in their cells and the ease of handling, fungi are thought to be great candidates for producing metal and metal sulfide nanoparticles ( Mohanpuria et al., 2008 ).
The nanoparticles were created on the surface of the mycelia. After analyzing the results and noting the solution, it was determined that the Ag + ions are initially trapped on the surface of the fungal cells by an electrostatic interaction between gold ions and negatively charged carboxylate groups, which is facilitated by enzymes that are present in the mycelia’s cell wall. Later, the enzymes in the cell wall reduce the silver ions, causing the development of silver nuclei. These nuclei then increase as more Ag ions are reduced and accumulate on them.
The TEM data demonstrate the presence of some silver nanoparticles both on and inside the cytoplasmic membrane. The findings concluded that the Ag ions that permeate through the cell wall were decreased by enzymes found inside the cytoplasm and on the cytoplasmic membrane. Also possible is the diffusion of some silver nanoparticles over the cell wall and eventual cytoplasmic entrapment ( Mukherjee et al., 2001 ; Hulkoti and Taranath, 2014 ).
It was observed that the culture’s age does not affect the shape of the synthesized gold nanoparticles. However, the number of particles decreased when older cells were used. The different pH levels produce a variety of shapes of gold nanoparticles, indicating that pH plays a vital role in determining the shape. The incubation temperature also played an essential role in the accumulation of the gold nanoparticles. It was observed that the particle growth rate was faster at increased temperature levels ( Mukherjee et al., 2001 ; Ahmad et al., 2003 ). The form of the produced gold nanoparticles was shown to be unaffected by the age of the culture. However, when older cells were utilized, the particle count fell. The fact that gold nanoparticles take on various forms at different pH levels suggests that the pH is crucial in determining the shape. The incubation temperature significantly influenced the accumulation of the gold nanoparticles. It was found that higher temperatures caused the particle development rate to accelerate ( Mukherjee et al., 2001 ; Ahmad et al., 2003 ). Verticillium luteoalbum is reported to synthesize gold nanoparticles of 20–40 nm in size ( Erasmus et al., 2014 ). Aspergillus terreus and Penicillium brevicompactum KCCM 60390 based metal NPs are reported for their antimicrobial ( Li G. et al., 2011 ) and cytotoxic activities ( Mishra et al., 2011 ), respectively.
Biosynthesis of NPs using actinomycetes: Actinomycetes have been categorized as prokaryotes since they share significant traits with fungi. They are sometimes referred to as ray fungi ( Mathew et al., 2010 ). Making NPs from actinomycetes is the same as that of fungi ( Sowani et al., 2016 ). Thermomonospora sp., a new species of extremophilic actinomycete, was discovered to produce extracellular, monodispersed, spherical gold nanoparticles with an average size of 8 nm ( Narayanan and Sakthivel, 2010 ). Metal NPs synthesized by Rhodococcus sp. ( Ahmad et al., 2003 ) and Streptomyces sp. Al-Dhabi-87 ( Al-Dhabi et al., 2018 ) are reported for their antimicrobial activities.
Biosynthesis of NPs using algae: Algae have a high concentration of polymeric molecules, and by reducing them, they may hyper-accumulate heavy metal ions and transform them into malleable forms. Algal extracts typically contain pigments, carbohydrates, proteins, minerals, polyunsaturated fatty acids, and other bioactive compounds like antioxidants that are used as stabilizing/capping and reducing agents ( Khanna et al., 2019 ). NPs also have a faster rate of photosynthesis than their biosynthetic counterparts. Live or dead algae are used as model organisms for the environmentally friendly manufacturing process of bio-nanomaterials, such as metallic NPs ( Hasan, 2015 ). Ag and Au are the most extensively researched noble metals to synthesized NPs by algae either intracellularly or extracellularly ( Dahoumane et al., 2017 ). Chlorella vulgaris ( Luangpipat et al., 2011 ), Chlorella pyrenoidosa ( Eroglu et al., 2013 ), Nanochloropsis oculata ( Xia et al., 2013 ), Scenedesmus sp. IMMTCC-25 ( Jena et al., 2014 ) based metal NPs are reported for their potential catalytic ( Luangpipat et al., 2011 ; Eroglu et al., 2013 ) and, antimicrobial ( Eroglu et al., 2013 ; Jena et al., 2014 ) activities along with their use in Li-Ion batteries ( Xia et al., 2013 ).
Intracellular synthesis of NPs using algae: In order to create intracellular NPs, algal biomass must first be gathered and thoroughly cleaned with distilled water. After that, the biomass (living algae) is treated with metallic solutions like AgNO3. The combination is then incubated at a specified pH and a specific temperature for a predetermined time. Finally, it is centrifuged and sonicated to produce the extracted stable NPs ( Uzair et al., 2020 ).
Extracellular synthesis of NPs using algae: Algal biomass is first collected and cleaned with distilled water before being used to synthesize NPs extracellularly ( Uzair et al., 2020 ). The following three techniques are frequently utilized for the subsequent procedure:
(i) A particular amount of time is spent drying the algal biomass (dead algae), after which the dried powder is treated with distilled water and filtered.
(ii) The algal biomass is sonicated with distilled water to get a cell-free extract.
(iii) The resultant product is filtered after the algal biomass has been rinsed with distilled water and incubated for a few hours (8–16 h).
5.2.6.2. Biological synthesis using plant extracts
The substance or active ingredient of the desired quality extracted from plant tissue by treatment for a particular purpose is a plant extract ( Jadoun et al., 2021 ). Plant extracts are combined with a metal salt solution at room temperature to create nanoparticles. Within minutes, the response is finished. This method has been used to create nanoparticles of silver, gold, and many other metals ( Li X. et al., 2011 ). Nanoparticles are biosynthesized using a variety of plants. It is known that the kind of plant extract, its concentration, the concentration of the metal salt, the pH, temperature, and the length of contact time all have an impact on how quickly nanoparticles are produced as well as their number and other properties ( Mittal and Chisti, 2013 ). A leaf extract from Polyalthia longifolia was used to create silver nanoparticles, the average particle size was around 58 nm ( Kumar and Yadav, 2009 ; Kumar et al., 2016 ).
Acacia auriculiformis ( Saini et al., 2016 ), Anisomeles indica ( Govindarajan et al., 2016 ), Azadirachta indica ( Velusamy et al., 2015 ), Bergenia ciliate ( Phull et al., 2016 ), Clitoria ternatea , Solanum nigrum ( Krithiga et al., 2013 ), Coffea arabica ( Dhand et al., 2016 ), Coleus forskohlii ( Naraginti et al., 2016 ), Curculigo orchioides ( Kayalvizhi et al., 2016 ), Digitaria radicosa ( Kalaiyarasu et al., 2016 ), Dioscorea alata ( Pugazhendhi et al., 2016 ), Diospyros paniculata ( Rao et al., 2016 ), Elephantopus scaber ( Kharat and Mendhulkar, 2016 ), Emblica officinalis ( Ramesh et al., 2015 ), Euphorbia antiquorum L. ( Rajkuberan et al., 2017 ), Ficus benghalensis ( Nayak et al., 2016 ), Lantana camara ( Ajitha et al., 2015 ), Cinnamomum zeylanicum ( Soni and Sonam, 2014 ), and Parkia roxburghii ( Paul et al., 2016 ) are the few examples of plants which are reported for the green synthesis of metal NPs (i.e., AgNPs). These were evaluated for their antifilaria activity ( Saini et al., 2016 ), mosquitocidal activity ( Govindarajan et al., 2016 ), antibacterial activity ( Velusamy et al., 2015 ), catalytic activity ( Edison et al., 2016 ), antioxidant activity ( Phull et al., 2016 ), and Cytotoxicity ( Patil et al., 2017 ).
5.2.6.3. Biological synthesis using biomimetic
“Biomimetic synthesis” typically refers to chemical processes that resemble biological synthesis carried out by living things ( Dahoumane et al., 2017 ). In the biomimetic approach, proteins, enzymes, cells, viruses, pollen, and waste biomass are used to synthesize NPs. Two categories are used to classify biomimetic synthesis:
Functional biomimetic synthesis uses various materials and approaches to emulate particular characteristics of natural materials, structures, and systems ( Zan and Wu, 2016 ).
Process biomimetic synthesis is a technique that aims to create different desirable nanomaterials/structures by imitating the synthesis pathways, processes, or procedures of natural chemicals and materials/structures. For instance, several distinctive nano-superstructures (such as satellite structures, dendrimer-like structures, pyramids, cubes, 2D nanoparticle arrays, 3D AuNP tubes, etc.) have been put together in vitro by simulating the protein manufacturing process ( Zan and Wu, 2016 ).
6. Applications of NPs
6.1. applications of nps in environment industry.
Due to their tiny size and distinctive physical and chemical characteristics, NPs appeal to various environmental applications. The properties of nanoparticals and their advantages are illustrated in Figure 4 . The following are some possible NP uses in the environment.
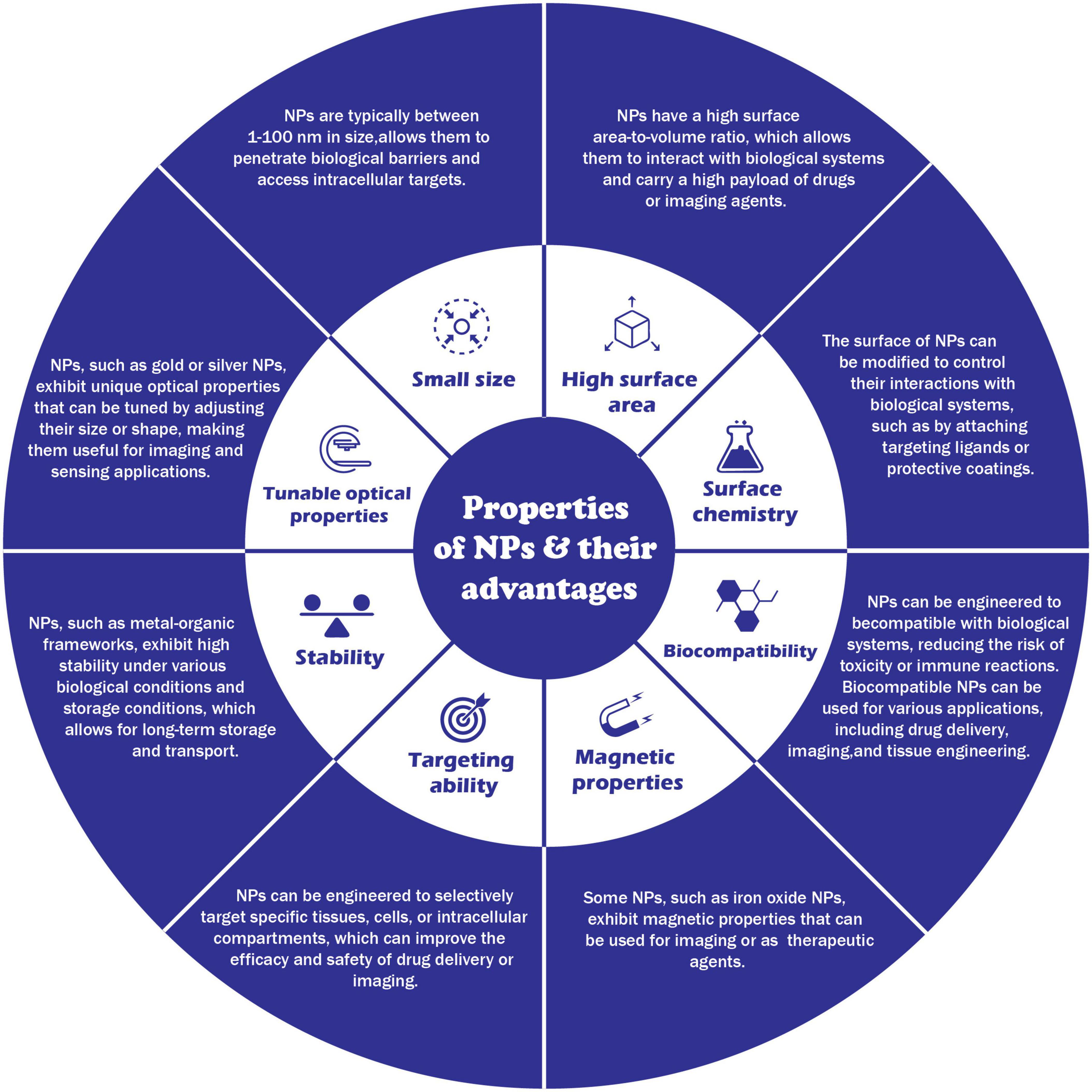
Figure 4. Properties of nanoparticals and their advantages.
6.1.1. Bioremediation
Nanoparticles (NPs) can remove environmental pollutants, such as heavy metals from water or organic contaminants from soil ( Zhuang and Gentry, 2011 ). For example, silver nanoparticles (AgNPs) effectively degrade certain pollutants, such as organic dyes and compounds found in wastewater. Several nanomaterials have been considered for remediation purposes, such as nanoscale zeolites, metal oxides, and carbon nanotubes and fibers ( Zhuang and Gentry, 2011 ). Nanoscale particles used in remediation can access areas that larger particles cannot. They can be coated to facilitate transport and prevent reaction with surrounding soil matrices before reacting with contaminants. One widely used nanomaterial for remediation is Nanoscale zerovalent iron (nZVI). It has been used at several hazardous waste sites to clean up chlorinated solvents that have contaminated groundwater ( Elliott et al., 2013 ). Removing heavy metals such as mercury, lead, thallium, cadmium, and arsenic from natural water has attracted considerable attention because of their adverse effects on environmental and human health. Superparamagnetic iron oxide NPs are an effective sorbent material for this toxic soft material. So, no measurements of engineered NPs in the environment have been available due to the absence of analytical methods able to quantify the trace concentration of NPs ( Elliott et al., 2013 ).
6.1.2. Sensors in environment
Nanotechnology/NPs are already being used to improve water quality and assist in environmental clean-up activities ( Pradeep, 2009 ). Their potential use as environmental sensors to monitor pollutants is also becoming viable NPs can be used as sensors to detect the presence of certain compounds in the environment, such as heavy metals or pollutants. The nano-sensors small size and wide detection range provide great flexibility in practical applications. It has been reported that nanoscale sensors can be used to detect microbial pathogens and biological compounds, such as toxins, in aqueous environments ( Yadav et al., 2010 ). NPS can be designed to selectively bind to specific types of pollutants, allowing them to be detected at low concentrations. For example, gold nanoparticles (AuNPs) have been used as sensors for the detection of mercury in water ( Theron et al., 2010 ).
6.1.3. Catalysts in environment
Nanoparticles (NPs) are used as catalysts in chemical reactions, such as in the production of biofuels or environmental remediation processes, and to catalyze biomass conversion into fuels, such as ethanol or biodiesel. For example, platinum nanoparticles (PtNPs) have been explored for use in the production of biofuels due to their ability to catalyze the conversion of biomass into fuels ( Lam and Luong, 2014 ). PtNPs also showed promising sensing properties; for example, Using Pt NPs, the Hg ions were quantified in the range of 50–500 nM in MilliQ, tap, and groundwater samples, and the limit of quantifications for Hg ions were 16.9, 26, and 47.3 nM. The biogenic PtNPs-based probe proved to be applicable for detecting and quantifying Hg ions ( Kora and Rastogi, 2018 ).
Overall, NPs have significant potential for use in the environment and are being actively researched for a variety of applications.
6.2. Applications of NPs in medicine industry
Nanoparticles (NPs) have unique physical and chemical properties due to their small size, making them attractive for use in various applications, including the medicine industry. Some potential applications of NPs in medicine include:
6.2.1. Drug delivery
Technological interest has been given to AuNPs due to their unique optical properties, ease of synthesis, and chemical stability. The particles can be used in biomedical applications such as cancer treatment ( Sun et al., 2014 ), biological imaging ( Abdulle and Chow, 2019 ), chemical sensing, and drug delivery. Sun et al. (2014) mentioned in detail about two different methods of controlled release of drugs associated with NPs, which were (1) sustained (i.e., diffusion-controlled and erosion-controlled) and (2) stimuli-responsive (i.e., pH-sensitive, enzyme-sensitive, thermoresponsive, and photosensitive). Figure 5 illustrates that how NPs acts as targeted delivery of medicines to treat cancer cells ( Figure 5A ) and therapeutic gene delivery to synthesis proteins of interests in targeted cells ( Figure 5B ). NPs can deliver drugs to specific body areas, allowing for more targeted and effective treatment ( Siddique and Chow, 2020 ). For example AgNPs have been explored for use in drug delivery due to their stability and ability to accumulate in certain types of cancerous tumors ( Siddique and Chow, 2020 ). ZnONPs have also been explored for drug delivery due to their ability to selectively target cancer cells ( Anjum et al., 2021 ). CuNPs have been shown to have antimicrobial properties and are being explored for drug delivery to treat bacterial infections ( Yuan et al., 2018 ). AuNPs have unique optical, electrical, and catalytic properties and are being explored for drug delivery due to their ability to accumulate in certain cancerous tumors. Silver NPs (AgNPs) have been incorporated into wound dressings, bone cement, and implants ( Schröfel et al., 2014 ).
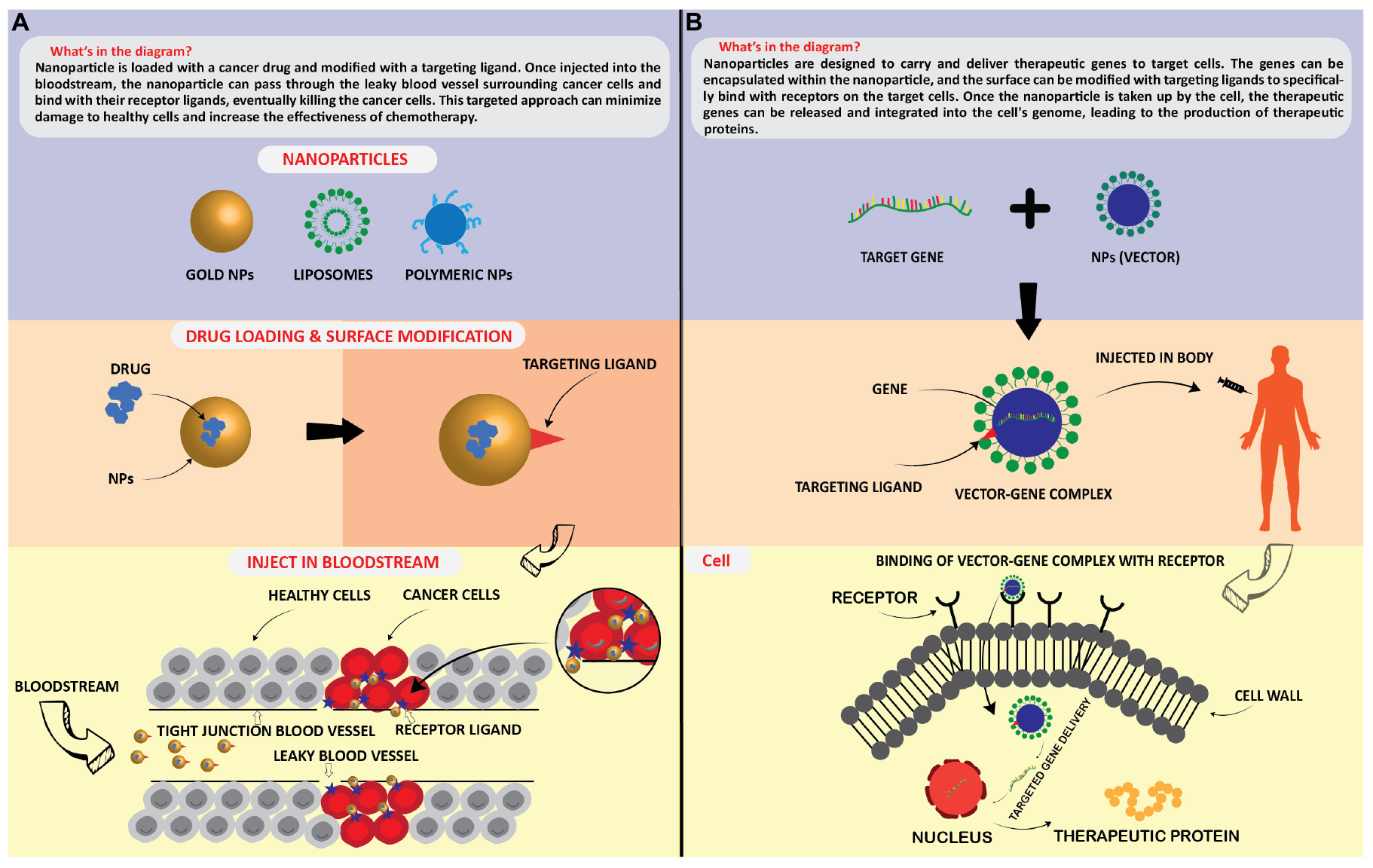
Figure 5. Application of nanoparticles as; targated drug delivery (A) , and therapeutic protein generation in targated cells (B) .
6.2.2. Diagnostics
Nanoparticles (NPs) can be used as imaging agents to help visualize specific body areas. For example, iron oxide nanoparticles (Fe 3 O 4 NPs) have been used as magnetic resonance imaging (MRI) contrast agents to help visualize tissues and organs ( Nguyen et al., 2013 ). AuNPs have unique optical, electrical, and catalytic properties and are being explored for diagnostics due to their ability to accumulate in certain cancerous tumors ( Siddique and Chow, 2020 ).
6.2.3. Tissue engineering
Nanoparticles (NPs) can help stimulate the growth and repair of tissues and organs. For example, titanium dioxide nanoparticles (TiO2 NPs) have been explored for tissue engineering due to their ability to stimulate the growth of bone cells ( Kim et al., 2014 ).
6.2.4. Antimicrobials
Some NPs, such as silver nanoparticles (AgNPs) and copper nanoparticles (CuNPs), have strong antimicrobial properties and are being explored for use in a variety of medical products, such as wound dressings and medical devices ( Hoseinzadeh et al., 2017 ).
Overall, NPs have significant potential for use in the medical industry and are being actively researched for various applications. However, it is essential to carefully consider the potential risks and benefits of using NPs in medicine and ensure their safe and responsible use.
6.3. Applications of NPs in agriculture industry
There are several ways in which nanoparticles (NPs) have the potential to alter the agricultural sector. NPs may be used in agriculture for a variety of reasons, including:
6.3.1. Pesticides and herbicides
Nanoparticles (NPs) can be used to deliver pesticides and herbicides in a targeted manner, reducing the number of chemicals needed and minimizing the potential for environmental contamination ( Khan et al., 2019 ). AgNPs and CuNPs have antimicrobial properties, making them potentially useful for controlling pests and diseases in crops. They can also be used as delivery systems for active ingredients, allowing for more targeted application and reducing the potential for environmental contamination ( Hoseinzadeh et al., 2017 ; Dangi and Verma, 2021 ).
It is important to note that using metal NPs in pesticides and herbicides is still in the early stages of development. More research is needed to understand their potential impacts on human health and the environment ( Dangi and Verma, 2021 ).
6.3.2. Fertilizers and plant growth
Nano fertilizers offer an opportunity for efficiently improving plant mineral nutrition. Some studies have shown that nanomaterials can be more effective than conventional fertilizers, with a controlled release of nutrients increasing the efficiency of plant uptake and potentially reducing adverse environmental outcomes associated with the loss of nutrients in the broader environment. However, other studies have found that nanomaterial has the same or even less effective effectiveness than conventional fertilizers. NPs used to deliver fertilizers to plants more efficiently, reducing the amount of fertilizer needed, and reducing the risk of nutrient runoff ( Kopittke et al., 2019 ).
Ag ( Jaskulski et al., 2022 ), Zn ( Song and Kim, 2020 ), Cu, Au, Al, and Fe ( Kopittke et al., 2019 ) based NPs have been shown to have fertilizing properties and plant growth-promoting properties, and may help provide essential nutrients to plants and improve plant growth and yield. It is important to note that the use of NPs in fertilizers is still in the early stages of development. More research is needed to understand their potential impacts on human health and the environment.
6.3.3. Food safety
Nanoparticles (NPs) can detect and eliminate pathogens in food products, improving food safety, and reducing the risk of foodborne illness ( Zhuang and Gentry, 2011 ).
6.3.4. Water purification
Nanoparticles (NPs) can purify irrigation water, reducing the risk of crop contamination and improving crop yield ( Zhuang and Gentry, 2011 ). Using NPs in agriculture can improve crop yields, reduce agriculture’s environmental impact, and improve food products’ safety and quality.
6.4. Applications of NPs in food industry
Numerous applications for nanoparticles (NPs) in the food sector are possible, including:
6.4.1. Food processing and food preservation/food packaging
Nanoparticles (NPs) can be used to improve the efficiency and performance of food processing operations, such as grinding, mixing, and drying, e.g., AgNPs have been used as a natural antimicrobial agent in food processing operations, helping to prevent the growth of bacteria and other microorganisms ( Dangi and Verma, 2021 ) and also NPs are used to enhance the performance of materials used in food packaging, making them more resistant to pollutants like moisture and gases.
6.4.2. Food fortification
Nanoparticles (NPs) can deliver essential nutrients to food products, such as vitamins and minerals, more efficiently and effectively. e.g., Fe 2 O 3 , and CuNPs have been used to fortify food products with iron, and Cu is an essential nutrient necessary for the metabolism of iron and other nutrients. Iron is an essential nutrient often lacking in many people’s diets, particularly in developing countries ( Kopittke et al., 2019 ).
6.4.3. Sensors
Nanoparticles (NPs) used to improve the sensitivity and specificity of food sensors, allowing them to detect a broader range of substances or signals ( Yadav et al., 2010 ).
Overall, using NPs in the food industry can improve the performance, safety, and nutritional value of a wide range of food products and processes.
6.5. Applications of NPs in electronics industry and automotive industry
In many aspects, nanoparticles (NPs) can transform the electronics sector. NPs may be used in a variety of electrical applications, such as:
6.5.1. Display technologies/storage devices
Nanoparticles (NPs) can be used to improve the performance of displays ( Park and Choi, 2019 ; Bahadur et al., 2021 ; Triana et al., 2022 ), such as LCD and OLED displays, by enhancing the brightness, color, and contrast of the image, such as silver NPs and gold NPs, have been explored for use in LCD and OLED displays as a means of improving the conductivity of the display ( Gwynne, 2020 ). NPs improve the performance and durability of energy storage devices, such as batteries and supercapacitors, by increasing energy density and charging speed. Zinc oxide nanoparticles (ZnO NPs) have the potential to be used in energy storage devices, such as batteries and supercapacitors, due to their ability to store and release energy ( Singh et al., 2011 ).
6.5.2. Data storage
Nanoparticles (NPs) can improve the capacity and speed of data storage devices, such as hard drives and flash drives. Magnetic NPs, such as iron oxide NPs, have been explored for use in data storage devices, such as hard drives, due to their ability to store, and retrieve data using magnetism. These NPs are often composed of a magnetic metal, such as iron, cobalt, or nickel. They can be magnetized and demagnetized, allowing them to store and retrieve data ( Ahmad et al., 2021 ).
Overall, the use of NPs in electronics has the potential to improve the performance and efficiency of a wide range of electronic devices and systems.
Applications of NPs in chemical industry: The chemical industry might be entirely transformed by nanoparticles (NPs) in various ways. The following are potential uses for NPs in the chemical industry ( Salem and Fouda, 2021 ).
6.5.3. Chemical processing/catalysis
Nanoparticles (NPs) can be used as catalysts in chemical reactions, allowing them to be carried out more efficiently and at lower temperatures. Some examples of metal NPs that have been used as catalysts in the chemical industry include: PtNPs have been used as catalysts in a variety of chemical reactions, including fuel cell reactions ( Bhavani et al., 2021 ), hydrogenation reactions, and oxidation reactions ( Lara and Philippot, 2014 ), PdNPs have been used as catalysts in a variety of chemical reactions, including hydrogenation reactions and cross-coupling reactions ( Pérez-Lorenzo, 2012 ), FeNPs have been used as catalysts in a variety of chemical reactions, including hydrolysis reactions ( Jiang and Xu, 2011 ), and oxygen reduction reactions, NiNPs have been used as catalysts in a variety of chemical reactions, including hydrogenation reactions, and hydrolysis reactions ( Salem and Fouda, 2021 ).
6.5.4. Separation and purification
NPs are used to separate and purify chemicals and other substances, such as gases and liquids, by exploiting their size-based properties ( Hollamby et al., 2010 ). Several types of metal nanoparticles (NPs) have been explored for use in separation and purification processes in the chemical industry, including Fe 2 O 3 NPs have been used to separate and purify gases, liquids, and chemicals. They have also been used to remove contaminants from water ( Pradeep, 2009 ; Siddique and Chow, 2020 ). AgNPs have been used to purify water and remove contaminants ( Pradeep, 2009 ), such as bacteria and viruses. They have also been used to remove heavy metals from water and other substances ( Zhuang and Gentry, 2011 ). AuNPs have been used to purify water and remove contaminants, such as bacteria and viruses ( Siddique and Chow, 2020 ). They have also been used to separate and purify gases and liquids ( Zhuang and Gentry, 2011 ). AlNPs have been used to remove contaminants from water and other substances, such as oils and fuels. They have also been used to purify gases ( Zhuang and Gentry, 2011 ).
6.6. Applications of NPs in defense industry
Nanoparticles (NPs) can be used to improve the efficiency and performance of chemical processing operations, such as refining and synthesizing chemicals ( Schröfel et al., 2014 ). Nanoparticles (NPs) have the potential to be used in the defense industry in several ways, including:
6.6.1. Sensors
Nanoparticles (NPs) can improve the sensitivity and specificity of sensors used in defense systems, such as sensors for detecting chemical, biological, or radiological threats ( Zheng et al., 2010 ).
6.6.2. Protective coatings
Nanoparticles (NPs) can improve the performance and durability of protective coatings applied to defense equipment, such as coatings resistant to chemical or biological agents. For example, metal NPs can improve the mechanical properties and durability of the coating, making it more resistant to wear and corrosion. For example, adding Al or Zn based NPs to a polymer coating can improve its corrosion resistance. In contrast, adding Ni or Cr-based NPs can improve their wear resistance ( Rangel-Olivares et al., 2021 ).
6.6.3. Weapons
Nanoparticles (NPs) are used as weapons against viruses, bacteria, etc, ( Ye et al., 2020 ) and as well as in the development of armor and protective materials. There have been some reports of the potential use of NPs in military and defense applications, such as in the development of armor and protective materials. For example, adding nanoparticles, such as ceramic or metal NPs, to polymers or other materials can improve their mechanical properties and make them more resistant to damage. In addition, there have been reports of the use of NPs in developing sensors and detection systems for defense purposes.
6.6.4. Manufacturing
Nanoparticles (NPs) can improve the performance and durability of materials used in defense equipment, such as armor or structural materials. Metal NPs can be used in materials by adding them as a filler or reinforcement in polymers. For example, the addition of metal NPs such as aluminum (Al), copper (Cu), or nickel (Ni) to polymers can improve the mechanical properties, thermal stability, and electrical conductivity of the resulting composite material ( Khan et al., 2019 ).
Metal NPs can also make functional materials, such as catalysts and sensors. For example, metal NPs, such as gold (Au), and platinum (Pt), can be used as catalysts in various chemical reactions due to their high surface area and ability to adsorb reactants ( Zheng et al., 2010 ).
6.6.5. Energy storage
Nanoparticles (NPs) can improve the performance and efficiency of energy storage systems used in defense systems, such as batteries or fuel cells ( Morsi et al., 2022 ). In batteries, nanoparticles can be used as a cathode material to increase the battery’s energy density, rate capability, and cycling stability. For example, lithium cobalt oxide (LiCoO 2 ) nanoparticles have been used as cathode materials in lithium-ion batteries due to their high capacity and good rate performance. In addition, nanoparticles of transition metal oxides, such as iron oxide (Fe 2 O 3 ), and manganese oxide (MnO 2 ), have been used as cathode materials in rechargeable lithium batteries due to their high capacity and good rate performance. In supercapacitors, nanoparticles can be used as the active material in the electrodes to increase the specific surface area, leading to an increase in the device’s capacitance ( Morsi et al., 2022 ). Using NPs in the defense industry can improve defense systems’ performance, efficiency, and safety.
7. Future perspectives
Metal nanoparticles (NPs) have many potential applications in various fields, including electronics, energy storage, catalysis, and medicine. However, there are also several challenges and potential future directions for developing and using metal NPs.
One major challenge is synthesizing and processing metal NPs with precise size and shape control. Many methods for synthesizing metal NPs involve high temperatures and harsh chemical conditions, which can be challenging to scale up for large-scale production. In addition, the size and shape of metal NPs can significantly impact their properties and potential applications, so it is essential to synthesize NPs with precise size and shape control.
Another challenge is the environmental impact of metal NPs. Some metal NPs, such as silver NPs, can be toxic to aquatic life and may have other environmental impacts. There is a need for more research on the environmental effects of metal NPs and the development of more environmentally friendly (Green) synthesis and processing methods.
In terms of future directions, one promising area is the use of metal NPs for energy storage, conversion, and protection of the environment. For example, metal NPs could be used to improve batteries’ performance or develop more efficient solar cells. In addition, metal NPs could be used in catalysis to improve the efficiency of chemical reactions. There is also ongoing research on metal NPs in medicine, including drug delivery and cancer therapy.
Author contributions
KAA: conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, writing – review and editing, and visualization.
Acknowledgments
The author thanks Prof. Dr. Mona M. Sobhy, Department of Reproductive Diseases, Animal Reproduction Research Institute, ARC, Giza, Egypt, and Dr. Omar Hewedy, University of Guelph, Canada, for the critical reading of the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdulle, A., and Chow, J. C. (2019). Contrast enhancement for portal imaging in nanoparticle-enhanced radiotherapy: A Monte Carlo phantom evaluation using flattening-filter-free photon beams. Nanomaterials 9:920. doi: 10.3390/nano9070920
PubMed Abstract | CrossRef Full Text | Google Scholar
Ago, H. (2015). “CVD growth of high-quality single-layer graphene,” in Frontiers of Graphene and Carbon Nanotubes , Ed. K. Matsumoto (Berlin: Springer), 3–20. doi: 10.1007/978-4-431-55372-4_1
CrossRef Full Text | Google Scholar
Ahmad, A., Alsaad, A., Al-Bataineh, Q. M., Al-Akhras, M.-A. H., Albataineh, Z., Alizzy, K. A., et al. (2021). Synthesis and characterization of ZnO NPs-doped PMMA-BDK-MR polymer-coated thin films with UV curing for optical data storage applications. Polymer Bull. 78, 1189–1211. doi: 10.1007/s00289-020-03155-x
Ahmad, A., Senapati, S., Khan, M. I., Kumar, R., Ramani, R., Srinivas, V., et al. (2003). Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete. Rhodococcus species. Nanotechnology 14:824. doi: 10.1088/0957-4484/14/7/323
Ahmad, T., Wani, I. A., Ahmed, J., and Al-Hartomy, O. A. (2014). Effect of gold ion concentration on size and properties of gold nanoparticles in TritonX-100 based inverse microemulsions. Appl. Nanosci. 4, 491–498. doi: 10.1007/s13204-013-0224-y
Ajitha, B., Reddy, Y. A. K., and Reddy, P. S. (2015). Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng. C 49, 373–381. doi: 10.1016/j.msec.2015.01.035
Al-Dhabi, N. A., Mohammed Ghilan, A.-K., and Arasu, M. V. (2018). Characterization of silver nanomaterials derived from marine Streptomyces sp. al-dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase clinical pathogens. Nanomaterials 8:279. doi: 10.3390/nano8050279
Amendola, V., and Meneghetti, M. (2009). Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 11, 3805–3821. doi: 10.1039/b900654k
Anjum, S., Hashim, M., Malik, S. A., Khan, M., Lorenzo, J. M., Abbasi, B. H., et al. (2021). Recent advances in zinc oxide nanoparticles (Zno nps) for cancer diagnosis, target drug delivery, and treatment. Cancers 13:4570. doi: 10.3390/cancers13184570
Astefanei, A., Núñez, O., and Galceran, M. T. (2015). Characterisation and determination of fullerenes: a critical review. Anal. Chim. Acta 882, 1–21.
Google Scholar
Bahadur, P. S., Jaiswal, S., Srivastava, R., and Kumar, A. (2021). “Advanced application of nanotechnology in engineering,” in Proceedings of the 2021 International Conference on Technological Advancements and Innovations (ICTAI) , (Piscataway, NJ: IEEE), 92–95.
Baig, N., Kammakakam, I., and Falath, W. (2021). Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2, 1821–1871.
Banerjee, A., Krishna, R., and Das, B. (2008). Size controlled deposition of Cu and Si nano-clusters by an ultra-high vacuum sputtering gas aggregation technique. Appl. Phys. A 90, 299–303.
Bayda, S., Adeel, M., Tuccinardi, T., Cordani, M., and Rizzolio, F. (2019). The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules 25:112. doi: 10.3390/molecules25010112
Behrisch, R. (1981). Sputtering by Particle Bombardment Springer Verlag. Berlin-Heidelberg: Springer.
Berkmans, A. J., Jagannatham, M., Priyanka, S., and Haridoss, P. (2014). Synthesis of branched, nano channeled, ultrafine and nano carbon tubes from PET wastes using the arc discharge method. Waste Manag. 34, 2139–2145. doi: 10.1016/j.wasman.2014.07.004
Beyene, H. D., Werkneh, A. A., Bezabh, H. K., and Ambaye, T. G. (2017). Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 13, 18–23.
Bhattacharjee, S. (2016). DLS and zeta potential–what they are and what they are not? J. Control. Release 235, 337–351.
Bhavani, K. S., Anusha, T., and Brahman, P. K. (2021). Platinum nanoparticles decorated on graphitic carbon nitride-ZIF-67 composite support: An electrocatalyst for the oxidation of butanol in fuel cell applications. Int. J. Hydr. Energy 46, 9199–9214.
Biju, V., Itoh, T., Anas, A., Sujith, A., and Ishikawa, M. (2008). Semiconductor quantum dots and metal nanoparticles: syntheses, optical properties, and biological applications. Anal. Bioanal. Chem. 391, 2469–2495.
Brady, B., Wang, P. H., Steenhoff, V., and Brolo, A. G. (2019). “Nanostructuring solar cells using metallic nanoparticles,” in Metal Nanostructures for Photonics , eds L. R. P. Kassab, and C. B. De Araujo (Amsterdam: Elsevier), 197–221.
Cadene, A., Durand-Vidal, S., Turq, P., and Brendle, J. (2005). Study of individual Na-montmorillonite particles size, morphology, and apparent charge. J. Colloid Interf. Sci. 285, 719–730. doi: 10.1016/j.jcis.2004.12.016
Chen, J., and Zhu, X. (2016). Magnetic solid phase extraction using ionic liquid-coated core–shell magnetic nanoparticles followed by high-performance liquid chromatography for determination of Rhodamine B in food samples. Food Chem. 200, 10–15. doi: 10.1016/j.foodchem.2016.01.002
Chen, J., Guo, Y., Zhang, X., Liu, J., Gong, P., Su, Z., et al. (2023). Emerging nanoparticles in food: sources, application, and safety. J. Agricult. Food Chem. 71, 3564–3582.
Chen, J., Wei, S., and Xie, H. (2021). “A brief introduction of carbon nanotubes: history, synthesis, and properties,” in Proceedings of the Journal of Physics: Conference Series , (United Kingdom: IOP Publishing), 012184. doi: 10.1088/1742-6596/1948/1/012184
Chen, J.-C., and Tang, C.-T. (2007). Preparation and application of granular ZnO/Al2O3 catalyst for the removal of hazardous trichloroethylene. J. Hazardous Mater. 142, 88–96. doi: 10.1016/j.jhazmat.2006.07.061
Chronakis, I. S. (2010). Micro-/nano-fibers by electrospinning technology: processing, properties and applications. Micromanufact. Eng. Technol. 2010, 264–286. doi: 10.1016/B978-0-8155-1545-6.00016-8
Compostella, F., Pitirollo, O., Silvestri, A., and Polito, L. (2017). Glyco-gold nanoparticles: synthesis and applications. Beilstein J. Org. Chem. 13, 1008–1021. doi: 10.3762/bjoc.13.100
Dahoumane, S. A., Mechouet, M., Wijesekera, K., Filipe, C. D., Sicard, C., Bazylinski, D. A., et al. (2017). Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology–a review. Green Chem. 19, 552–587. doi: 10.1039/C6GC02346K
Dangi, K., and Verma, A. K. (2021). Efficient & eco-friendly smart nano-pesticides: Emerging prospects for agriculture. Mater. Today Proc. 45, 3819–3824.
Das, S., and Srivasatava, V. C. (2016). Synthesis and characterization of ZnO–MgO nanocomposite by co-precipitation method. Smart Sci. 4, 190–195.
De La Calle, I., Menta, M., Klein, M., and Séby, F. (2018). Study of the presence of micro-and nanoparticles in drinks and foods by multiple analytical techniques. Food Chem. 266, 133–145. doi: 10.1016/j.foodchem.2018.05.107
Delvallée, A., Feltin, N., Ducourtieux, S., Trabelsi, M., and Hochepied, J. (2015). Direct comparison of AFM and SEM measurements on the same set of nanoparticles. Measur. Sci. Technol. 26:085601.
Dhand, V., Soumya, L., Bharadwaj, S., Chakra, S., Bhatt, D., and Sreedhar, B. (2016). Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C 58, 36–43. doi: 10.1016/j.msec.2015.08.018
Dikusar, A., Globa, P., Belevskii, S., and Sidel’nikova, S. (2009). On limiting rate of dimensional electrodeposition at meso-and nanomaterial manufacturing by template synthesis. Surf. Eng. Appl. Electrochem. 45, 171–179.
Dragovic, R. A., Gardiner, C., Brooks, A. S., Tannetta, D. S., Ferguson, D. J., Hole, P., et al. (2011). Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine 7, 780–788.
Dreaden, E. C., Alkilany, A. M., Huang, X., Murphy, C. J., and El-Sayed, M. A. (2012). The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 41, 2740–2779. doi: 10.1039/c1cs15237h
Du, P., Song, L., Xiong, J., Li, N., Xi, Z., Wang, L., et al. (2012). Coaxial electrospun TiO2/ZnO core–sheath nanofibers film: Novel structure for photoanode of dye-sensitized solar cells. Electrochim. Acta 78, 392–397.
Edison, T. N. J. I., Lee, Y. R., and Sethuraman, M. G. (2016). Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow-12 dye. Pectrochimica Acta Part A 161, 122–129. doi: 10.1016/j.saa.2016.02.044
Elliott, J. A., Shibuta, Y., Amara, H., Bichara, C., and Neyts, E. C. (2013). Atomistic modelling of CVD synthesis of carbon nanotubes and graphene. Nanoscale 5, 6662–6676. doi: 10.1039/c3nr01925j
Erasmus, M., Cason, E. D., Van Marwijk, J., Botes, E., Gericke, M., and Van Heerden, E. (2014). Gold nanoparticle synthesis using the thermophilic bacterium Thermus scotoductus SA-01 and the purification and characterization of its unusual gold reducing protein. Gold Bull. 47, 245–253.
Eroglu, E., Chen, X., Bradshaw, M., Agarwal, V., Zou, J., Stewart, S. G., et al. (2013). Biogenic production of palladium nanocrystals using microalgae and their immobilization on chitosan nanofibers for catalytic applications. RSC Adv. 3, 1009–1012.
Essajai, R., Benhouria, Y., Rachadi, A., Qjani, M., Mzerd, A., and Hassanain, N. (2019). Shape-dependent structural and magnetic properties of Fe nanoparticles studied through simulation methods. RSC Adv. 9, 22057–22063. doi: 10.1039/c9ra03047f
Falke, S., and Betzel, C. (2019). “Dynamic light scattering (DLS),” in Radiation in Bioanalysis , eds A. S. Pereira, P. Tavares, P. Limão-Vieira (Berlin: Springer), 173–193.
Farrell, D., Majetich, S. A., and Wilcoxon, J. P. (2003). Preparation and characterization of monodisperse Fe nanoparticles. J. Phys. Chem. B 107, 11022–11030.
Feng, L., Xuan, Z., Ma, J., Chen, J., Cui, D., Su, C., et al. (2015). Preparation of gold nanorods with different aspect ratio and the optical response to solution refractive index. J. Exp. Nanosci. 10, 258–267.
Ghorbani, H. R., Mehr, F. P., Pazoki, H., and Rahmani, B. M. (2015). Synthesis of ZnO nanoparticles by precipitation method. Orient. J. Chem. 31, 1219–1221.
Ghosh, S., Ahmad, R., Zeyaullah, M., and Khare, S. K. (2021). Microbial nano-factories: synthesis and biomedical applications. Front. Chem. 9:194. doi: 10.3389/fchem.2021.626834
Giljohann, D. A., Seferos, D. S., Daniel, W. L., Massich, M. D., Patel, P. C., and Mirkin, C. A. (2020). Gold nanoparticles for biology and medicine. Spherical Nucleic Acids 49, 3280–3294.
Giurlani, W., Innocenti, M., and Lavacchi, A. (2018). X-ray microanalysis of precious metal thin films: thickness and composition determination. Coatings 8:84.
Gloria, E. C., Ederley, V., Gladis, M., César, H., Jaime, O., Oscar, A., et al. (2017). “Synthesis of silver nanoparticles (AgNPs) with antibacterial activity,” in Proceedings of the Journal of Physics: Conference Series , (United Kingdom: IOP Publishing), 012023.
Gorrasi, G., and Sorrentino, A. (2015). Mechanical milling as a technology to produce structural and functional bio-nanocomposites. Green Chem. 17, 2610–2625.
Govindarajan, M., Rajeswary, M., Veerakumar, K., Muthukumaran, U., Hoti, S., and Benelli, G. (2016). Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp. Parasitol. 161, 40–47. doi: 10.1016/j.exppara.2015.12.011
Graf, C., Vossen, D. L., Imhof, A., and Van Blaaderen, A. (2003). A general method to coat colloidal particles with silica. Langmuir 19, 6693–6700.
Greczynski, G., and Hultman, L. (2020). X-ray photoelectron spectroscopy: towards reliable binding energy referencing. Progr. Mater. Sci. 107:100591.
Guo, D., Xie, G., and Luo, J. (2013). Mechanical properties of nanoparticles: basics and applications. J. Phys. D 47:013001.
Guo, W., Pleixats, R., and Shafir, A. (2015). Water-soluble gold nanoparticles: from catalytic selective Nitroarene reduction in water to refractive index sensing. Chem. An Asian J. 10, 2437–2443. doi: 10.1002/asia.201500290
Gwynne, K. (2020). Enhancement of the Photostability of Blue Phosphorescence Using Plasmonic Surfaces. New Brunswick, NJ: Rutgers University-School of Graduate Studies.
Haasch, R. T. (2014). “X-ray photoelectron spectroscopy (XPS) and auger electron spectroscopy (AES),” in Practical Materials Characterization , Ed. M. Sardela (Berlin: Springer), 93–132.
Hasan, S. (2015). A review on nanoparticles: their synthesis and types. Res. J. Recent Sci. 2277:2502.
Holder, C. F., and Schaak, R. E. (2019). Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. Washington, DC: ACS Publications.
Hollamby, M. J., Eastoe, J., Chemelli, A., Glatter, O., Rogers, S., Heenan, R. K., et al. (2010). Separation and purification of nanoparticles in a single step. Langmuir 26, 6989–6994. doi: 10.1021/la904225k
Hoo, C. M., Starostin, N., West, P., and Mecartney, M. L. (2008). A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanopart. Res. 10, 89–96.
Hortin, J., Anderson, A., Britt, D., Jacobson, A., and Mclean, J. (2020). Copper oxide nanoparticle dissolution at alkaline pH is controlled by dissolved organic matter: influence of soil-derived organic matter, wheat, bacteria, and nanoparticle coating. Environ. Sci. 7, 2618–2631.
Hoseinzadeh, E., Makhdoumi, P., Taha, P., Hossini, H., Stelling, J., and Amjad Kamal, M. (2017). A review on nano-antimicrobials: metal nanoparticles, methods and mechanisms. Curr. Drug Metab. 18, 120–128.
PubMed Abstract | Google Scholar
Hulkoti, N. I., and Taranath, T. (2014). Biosynthesis of nanoparticles using microbes—a review. Colloids Surf. B Biointerf. 121, 474–483. doi: 10.1016/j.colsurfb.2014.05.027
Islam, F., Shohag, S., Uddin, M. J., Islam, M. R., Nafady, M. H., Akter, A., et al. (2022). Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials 15:2160. doi: 10.3390/ma15062160
Jadoun, S., Arif, R., Jangid, N. K., and Meena, R. K. (2021). Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 19, 355–374.
Jamkhande, P. G., Ghule, N. W., Bamer, A. H., and Kalaskar, M. G. (2019). Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 53, 101174.
Jana, N. R., Earhart, C., and Ying, J. Y. (2007). Synthesis of water-soluble and functionalized nanoparticles by silica coating. Chem. Mater. 19, 5074–5082.
Jaskulski, D., Jaskulska, I., Majewska, J., Radziemska, M., Bilgin, A., and Brtnicky, M. (2022). Silver Nanoparticles (AgNPs) in urea solution in laboratory tests and field experiments with crops and vegetables. Materials 15:870. doi: 10.3390/ma15030870
Jayaraman, V., Ghosh, S., Sengupta, A., Srivastava, S., Sonawat, H., and Narayan, P. K. (2014). Identification of biochemical differences between different forms of male infertility by nuclear magnetic resonance (NMR) spectroscopy. J. Assist. Reproduct. Genet. 31, 1195–1204. doi: 10.1007/s10815-014-0282-4
Jena, J., Pradhan, N., Nayak, R. R., Dash, B. P., Sukla, L. B., Panda, P. K., et al. (2014). Microalga Scenedesmus sp.: a potential low-cost green machine for silver nanoparticle synthesis. J. Microbiol. Biotechnol. Adv. 24, 522–533. doi: 10.4014/jmb.1306.06014
Jiang, H.-L., and Xu, Q. (2011). Catalytic hydrolysis of ammonia borane for chemical hydrogen storage. Catal. Today 170, 56–63.
Joh, D.-W., Jung, T.-K., Lee, H.-S., and Kim, D.-H. (2013). Synthesis of nanoparticles using electrical explosion of Ni wire in Pt solution. J. Nanosci. Nanotechnol. 13, 6092–6094. doi: 10.1166/jnn.2013.7677
Kahle, M., Kleber, M., and Jahn, R. (2002). Review of XRD-based quantitative analyses of clay minerals in soils: the suitability of mineral intensity factors. Geoderma 109, 191–205.
Kalaiyarasu, T., Karthi, N., Sharmila, G. V., and Manju, V. (2016). In vitro assessment of antioxidant and antibacterial activity of green synthesized silver nanoparticles from Digitaria radicosa leaves. Asian J. Pharm. Clin. Res. 9, 297–302.
Kayalvizhi, T., Ravikumar, S., and Venkatachalam, P. (2016). Green synthesis of metallic silver nanoparticles using Curculigo orchioides rhizome extracts and evaluation of its antibacterial, larvicidal, and anticancer activity. J. Environ. Eng. 142:C4016002.
Khan, A., Rashid, R., Murtaza, G., and Zahra, A. (2014). Gold nanoparticles: synthesis and applications in drug delivery. Trop. J. Pharm. Res. 13, 1169–1177.
Khan, I., Saeed, K., and Khan, I. (2019). Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 12, 908–931.
Khanna, P., Kaur, A., and Goyal, D. (2019). Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol Methods 163:105656. doi: 10.1016/j.mimet.2019.105656
Kharat, S. N., and Mendhulkar, V. D. (2016). Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C 62, 719–724. doi: 10.1016/j.msec.2016.02.024
Kim, J.-H., Sheikh, F. A., Ju, H. W., Park, H. J., Moon, B. M., Lee, O. J., et al. (2014). 3D silk fibroin scaffold incorporating titanium dioxide (TiO2) nanoparticle (NPs) for tissue engineering. Int. J. Biol. Macromol. 68, 158–168. doi: 10.1016/j.ijbiomac.2014.04.045
Kohl, H., and Reimer, L. (2008). Transmission Electron Microscopy. Berlin: Springer Series in Optical Sciences, 36.
Kokarneswaran, M., Selvaraj, P., Ashokan, T., Perumal, S., Sellappan, P., Murugan, K. D., et al. (2020). Discovery of carbon nanotubes in sixth century BC potteries from Keeladi, India. Sci. Rep. 10, 1–6. doi: 10.1038/s41598-020-76720-z
Kopittke, P. M., Lombi, E., Wang, P., Schjoerring, J. K., and Husted, S. (2019). Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. 6, 3513–3524. doi: 10.3390/biology10111123
Kora, A. J., and Rastogi, L. (2018). Peroxidase activity of biogenic platinum nanoparticles: A colorimetric probe towards selective detection of mercuric ions in water samples. Sens. Actuators B Chem. 254, 690–700.
Kreizer, M., Ratner, D., and Liberzon, A. (2010). Real-time image processing for particle tracking velocimetry. Exp. Fluids 48, 105–110.
Krithiga, N., Jayachitra, A., and Rajalakshmi, A. (2013). Synthesis, characterization and analysis of the effect of copper oxide nanoparticles in biological systems. Ind. J. Ns 1, 6–15.
Kumar, R., Singh, R. K., Dubey, P. K., Kumar, P., Tiwari, R. S., and Oh, I.-K. (2013). Pressure-dependent synthesis of high-quality few-layer graphene by plasma-enhanced arc discharge and their thermal stability. J. Nanopart. Res. 15, 1–10.
Kumar, S. S., Venkateswarlu, P., Rao, V. R., and Rao, G. N. (2013). Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 3, 1–6.
Kumar, V., and Yadav, S. K. (2009). Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 84, 151–157.
Kumar, V., Bano, D., Mohan, S., Singh, D. K., and Hasan, S. H. (2016). Sunlight-induced green synthesis of silver nanoparticles using aqueous leaf extract of Polyalthia longifolia and its antioxidant activity. Mater. Lett. 181, 371–377.
Kumari, S. C., Dhand, V., and Padma, P. N. (2021). Green synthesis of metallic nanoparticles: a review. Nanomaterials 2021, 259–281.
Lam, E., and Luong, J. H. (2014). Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 4, 3393–3410.
Lara, P., and Philippot, K. (2014). The hydrogenation of nitroarenes mediated by platinum nanoparticles: an overview. Catal. Sci. Technol. 4, 2445–2465.
Lerner, M. I., Glazkova, E. A., Lozhkomoev, A. S., Svarovskaya, N. V., Bakina, O. V., Pervikov, A. V., et al. (2016). Synthesis of Al nanoparticles and Al/AlN composite nanoparticles by electrical explosion of aluminum wires in argon and nitrogen. Powder Technol. 295, 307–314.
Lewczuk, B., and Szyryńska, N. (2021). Field-emission scanning electron microscope as a tool for large-area and large-volume ultrastructural studies. Animals 11:3390. doi: 10.3390/ani11123390
Li, G., He, D., Qian, Y., Guan, B., Gao, S., Cui, Y., et al. (2011). Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 13, 466–476. doi: 10.3390/ijms13010466
Li, N., Zhao, P., and Astruc, D. (2014). Anisotropic gold nanoparticles: synthesis, properties, applications, and toxicity. Angewand. Chem. Int. Edn. 53, 1756–1789.
Li, T., Senesi, A. J., and Lee, B. (2016). Small angle X-ray scattering for nanoparticle research. Chem. Rev. 116, 11128–11180. doi: 10.1021/acs.chemrev.5b00690
Li, X., Xu, H., Chen, Z.-S., and Chen, G. (2011). Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011:270974.
Luangpipat, T., Beattie, I. R., Chisti, Y., and Haverkamp, R. G. (2011). Gold nanoparticles produced in a microalga. J. Nanopart. Res. 13, 6439–6445.
Lyon, L. A., Keating, C. D., Fox, A. P., Baker, B. E., He, L., Nicewarner, S. R., et al. (1998). Raman spectroscopy. Anal. Chem. 70, 341–362.
Lyu, H., Gao, B., He, F., Ding, C., Tang, J., and Crittenden, J. C. (2017). Ball-milled carbon nanomaterials for energy and environmental applications. ACS Sust. Chem. Eng. 5, 9568–9585. doi: 10.1016/j.biortech.2020.123613
Machac, P., Cichon, S., Lapcak, L., and Fekete, L. (2020). Graphene prepared by chemical vapour deposition process. Graph. Technol. 5, 9–17.
Madathil, A. N. P., Vanaja, K., and Jayaraj, M. (2007). “Synthesis of ZnO nanoparticles by hydrothermal method,” in Nanophotonic materials IV , eds Z. Gaburro and S. Cabrini (Bellingham, WA: SPIE), 47–55.
Maharani, V., Sundaramanickam, A., and Balasubramanian, T. J. E. (2016). In vitro anticancer activity of silver nanoparticle synthesized by Escherichia coli VM1 isolated from marine sediments of Ennore southeast coast of India. Enzyme Microb. Technol. 95, 146–154. doi: 10.1016/j.enzmictec.2016.09.008
Majeed Khan, M. A., Kumar, S., Ahamed, M., Alrokayan, S. A., and Alsalhi, M. S. (2011). Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanosc. Res. Lett. 6, 1–8.
Malhotra, S. P. K., and Alghuthaymi, M. A. (2022). Biomolecule-assisted biogenic synthesis of metallic nanoparticles. Agri-Waste Microb. Product. Sust. Nanomater. 2022, 139–163.
Mathew, L., Chandrasekaran, N., and Mukherjee, A. (2010). “Biomimetic synthesis of nanoparticles: science, technology & applicability,” Biomimetics learning from nature , Ed. A. Mukherjee (Norderstedt: Books on Demand).
Mishra, A., Tripathy, S. K., Wahab, R., Jeong, S.-H., Hwang, I., Yang, Y.-B., et al. (2011). Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse mayo blast cancer C 2 C 12 cells. Appl. Microbiol. Biotechnol. Adv. 92, 617–630. doi: 10.1007/s00253-011-3556-0
Mittal, A., and Chisti, Y. (2013). Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 31, 346–356.
Moghaddam, A. B., Nazari, T., Badraghi, J., and Kazemzad, M. (2009). Synthesis of ZnO nanoparticles and electrodeposition of polypyrrole/ZnO nanocomposite film. Int. J. Electrochem. Sci. 4, 247–257.
Mohanpuria, P., Rana, N. K., and Yadav, S. K. (2008). Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart. Res. 10, 507–517.
Mohd Yusof, H., Mohamad, R., Zaidan, U. H., and Rahman, A. (2019). Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J. Anim. Sci. Biotechnol. 10, 1–22. doi: 10.1186/s40104-019-0368-z
Morsi, M., Abdelrazek, E., Ramadan, R., Elashmawi, I., and Rajeh, A. (2022). Structural, optical, mechanical, and dielectric properties studies of carboxymethyl cellulose/polyacrylamide/lithium titanate nanocomposites films as an application in energy storage devices. Polymer Test. 114, 107705.
Mott, D., Galkowski, J., Wang, L., Luo, J., and Zhong, C.-J. (2007). Synthesis of size-controlled and shaped copper nanoparticles. Langmuir 23, 5740–5745.
Mukherjee, P., Ahmad, A., Mandal, D., Senapati, S., Sainkar, S. R., Khan, M. I., et al. (2001). Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 1, 515–519.
Muñoz-García, J., Vázquez, L., Cuerno, R., Sánchez-García, J. A., Castro, M., and Gago, R. (2009). “Self-organized surface nanopatterning by ion beam sputtering,” in Toward Functional Nanomaterials , Ed. Z. M. Wang (Berlin: Springer), 323–398.
Naraginti, S., Kumari, P. L., Das, R. K., Sivakumar, A., Patil, S. H., and Andhalkar, V. V. (2016). Amelioration of excision wounds by topical application of green synthesized, formulated silver and gold nanoparticles in albino Wistar rats. Mater. Sci. Eng. C 62, 293–300. doi: 10.1016/j.msec.2016.01.069
Narayanan, K. B., and Sakthivel, N. (2010). Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interf. Sci. 156, 1–13.
Nayak, D., Ashe, S., Rauta, P. R., Kumari, M., and Nayak, B. (2016). Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C 58, 44–52. doi: 10.1016/j.msec.2015.08.022
Ndolomingo, M. J., and Meijboom, R. (2016). Determination of the surface area and sizes of supported copper nanoparticles through organothiol adsorption—Chemisorption. Appl. Surf. Sci. 390, 224–235.
Newbury, D. E., and Ritchie, N. W. (2013). Is scanning electron microscopy/energy dispersive X-ray spectrometry (SEM/EDS) quantitative? Scanning 35, 141–168. doi: 10.1002/sca.21041
Nguyen, K. T., Menon, J. U., Jadeja, P. V., Tambe, P. P., Vu, K., and Yuan, B. (2013). Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics 3, 152–166.
Nowack, B., Krug, H. F., and Height, M. (2011). 120 Years of Nanosilver History: Implications for Policy Makers. Washington, DC: ACS Publications.
Önal, E. S., Yatkın, T., Aslanov, T., Ergüt, M., and Özer, A. (2019). Biosynthesis and characterization of iron nanoparticles for effective adsorption of Cr (VI). Int. J. Chem. Eng. 2019:2716423.
Ostermann, R., Cravillon, J., Weidmann, C., Wiebcke, M., and Smarsly, B. M. (2011). Metal–organic framework nanofibers via electrospinning. Chem. Commun. 47, 442–444.
Parashar, M., Shukla, V. K., and Singh, R. (2020). Metal oxides nanoparticles via sol–gel method: a review on synthesis, characterization and applications. J. Mater. Sci. 31, 3729–3749.
Park, C. Y., and Choi, B. (2019). Enhanced light extraction from bottom emission oleds by high refractive index nanoparticle scattering layer. Nanomaterials 9:1241. doi: 10.3390/nano9091241
Patil, M. P., and Kim, G.-D. (2018). Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerf. 172, 487–495.
Patil, M. P., Ngabire, D., Thi, H. H. P., Kim, M.-D., and Kim, G.-D. (2017). Eco-friendly synthesis of gold nanoparticles and evaluation of their cytotoxic activity on cancer cells. J. Clust. Sci. 28, 119–132.
Patil, N., Bhaskar, R., Vyavhare, V., Dhadge, R., Khaire, V., and Patil, Y. (2021). Overview on methods of synthesis of nanoparticles. Int. J. Curr. Pharm. Res. 13, 11–16.
Patois, E., Capelle, M., Palais, C., Gurny, R., and Arvinte, T. (2012). Evaluation of nanoparticle tracking analysis (NTA) in the characterization of therapeutic antibodies and seasonal influenza vaccines: pros and cons. J. Drug Deliv. Sci. Technol. 22, 427–433.
Paul, B., Bhuyan, B., Purkayastha, D. D., and Dhar, S. S. (2016). Photocatalytic and antibacterial activities of gold and silver nanoparticles synthesized using biomass of Parkia roxburghii leaf. J. Photochem. Photobiol. B Biol. 154, 1–7. doi: 10.1016/j.jphotobiol.2015.11.004
Pérez-Lorenzo, M. (2012). Palladium nanoparticles as efficient catalysts for Suzuki cross-coupling reactions. J. Phys. Chem. Lett. 3, 167–174.
Pérez-Tijerina, E., Pinilla, M. G., Mejía-Rosales, S., Ortiz-Méndez, U., Torres, A., and José-Yacamán, M. (2008). Highly size-controlled synthesis of Au/Pd nanoparticles by inert-gas condensation. Faraday Discuss. 138, 353–362. doi: 10.1039/b705913m
Phull, A.-R., Abbas, Q., Ali, A., Raza, H., Zia, M., and Haq, I.-U. (2016). Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Fut. J. Pharm. Sci. 2, 31–36.
Pimpin, A., and Srituravanich, W. (2012). Review on micro-and nanolithography techniques and their applications. Eng. J. 16, 37–56.
Pradeep, T. (2009). Noble metal nanoparticles for water purification: a critical review. Thin Solid Films 517, 6441–6478.
Praseptiangga, D., Zahara, H. L., Widjanarko, P. I., Joni, I. M., and Panatarani, C. (2020). Preparation and FTIR spectroscopic studies of SiO2-ZnO nanoparticles suspension for the development of carrageenan-based bio-nanocomposite film. 100005.
Pugazhendhi, S., Sathya, P., Palanisamy, P., and Gopalakrishnan, R. (2016). Synthesis of silver nanoparticles through green approach using Dioscorea alata and their characterization on antibacterial activities and optical limiting behavior. J. Photochem. Photobiol. B Biol. 159, 155–160. doi: 10.1016/j.jphotobiol.2016.03.043
Qi, P., Zhang, D., and Wan, Y. (2013). Sulfate-reducing bacteria detection based on the photocatalytic property of microbial synthesized ZnS nanoparticles. Anal. Chim. Acta 800, 65–70. doi: 10.1016/j.aca.2013.09.015
Rad, A. G., Abbasi, H., and Afzali, M. H. (2011). Gold nanoparticles: synthesising, characterizing and reviewing novel application in recent years. Phys. Proc. 22, 203–208.
Rahmati-Abkenar, M., and Manteghian, M. (2020). Effect of silver nanoparticles on the solubility of methane and ethane in water. J. Nat. Gas Sci. Eng. 82:103505.
Rajeshkumar, S., Ponnanikajamideen, M., Malarkodi, C., Malini, M., and Annadurai, G. (2014). Microbe-mediated synthesis of antimicrobial semiconductor nanoparticles by marine bacteria. J. Nanostruct. Chem. 4, 1–7.
Rajkuberan, C., Prabukumar, S., Sathishkumar, G., Wilson, A., Ravindran, K., and Sivaramakrishnan, S. (2017). Facile synthesis of silver nanoparticles using Euphorbia antiquorum L. latex extract and evaluation of their biomedical perspectives as anticancer agents. J. Saudi Chem. Soc. 21, 911–919.
Ramesh, P., Kokila, T., and Geetha, D. (2015). Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim. Acta Part A 142, 339–343. doi: 10.1016/j.saa.2015.01.062
Rangel-Olivares, F. R., Arce-Estrada, E. M., and Cabrera-Sierra, R. (2021). Synthesis and characterization of polyaniline-based polymer nanocomposites as anti-corrosion coatings. Coatings 11:653.
Rao, N. H., Lakshmidevi, N., Pammi, S., Kollu, P., Ganapaty, S., and Lakshmi, P. (2016). Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater. Sci. Eng. C 62, 553–557. doi: 10.1016/j.msec.2016.01.072
Rassaei, L., Marken, F., Sillanpää, M., Amiri, M., Cirtiu, C. M., and Sillanpää, M. (2011). Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal. Chem. 30, 1704–1715.
Rocha, F. S., Gomes, A. J., Lunardi, C. N., Kaliaguine, S., and Patience, G. S. (2018). Experimental methods in chemical engineering: Ultraviolet visible spectroscopy—UV-Vis. Can. J. Chem. Eng. 96, 2512–2517.
Saini, P., Saha, S. K., Roy, P., Chowdhury, P., and Babu, S. P. S. (2016). Evidence of reactive oxygen species (ROS) mediated apoptosis in Setaria cervi induced by green silver nanoparticles from Acacia auriculiformis at a very low dose. Exp. Parasitol. 160, 39–48. doi: 10.1016/j.exppara.2015.11.004
Saldarriaga, J. F., Aguado, R., Pablos, A., Amutio, M., Olazar, M., and Bilbao, J. (2015). Fast characterization of biomass fuels by thermogravimetric analysis (TGA). Fuel 140, 744–751.
Salem, S. S., and Fouda, A. (2021). Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol. Trace Element Res. 199, 344–370. doi: 10.1007/s12011-020-02138-3
Salopek, B., Krasic, D., and Filipovic, S. (1992). Measurement and application of zeta-potential. Rudarsko-Geolosko-Naftni Zbornik 4:147.
Saw, M. J., Ghosh, B., Nguyen, M. T., Jirasattayaporn, K., Kheawhom, S., Shirahata, N., et al. (2019). High aspect ratio and post-processing free silver nanowires as top electrodes for inverted-structured photodiodes. ACS Omega 4, 13303–13308. doi: 10.1021/acsomega.9b01479
Schröfel, A., Kratošová, G., Šafar̄ík, I., Šafar̄íková, M., Raška, I., and Shor, L. M. (2014). Applications of biosynthesized metallic nanoparticles–a review. Acta Biomater. 10, 4023–4042.
Shenashen, M. A., El-Safty, S. A., and Elshehy, E. A. (2014). Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part. Part. Syst. Char. 31, 293–316.
Shi, Z., Lian, Y., Liao, F. H., Zhou, X., Gu, Z., Zhang, Y., et al. (2000). Large scale synthesis of single-wall carbon nanotubes by arc-discharge method. J. Phys. Chem. Solids 61, 1031–1036. doi: 10.1166/jnn.2001.012
Siddiqi, K. S., and Husen, A. (2016). Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanosc. Res. Lett. 11, 1–13. doi: 10.1186/s11671-016-1695-z
Siddique, S., and Chow, J. C. (2020). Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 10:3824.
Sigmund, W., Yuh, J., Park, H., Maneeratana, V., Pyrgiotakis, G., and Daga, A. (2006). Processing and structure relationships in electrospinning of ceramic fiber systems. J. Am. Ceramic Soc. 89, 395–407.
Singh, R. P., Shukla, V. K., Yadav, R. S., Sharma, P. K., Singh, P. K., and Pandey, A. C. (2011). Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett. 2, 313–317.
Siwach, O. P., and Sen, P. (2008). Synthesis and study of fluorescence properties of Cu nanoparticles. J. Nanopart. Res. 10, 107–114.
Slavin, Y. N., Asnis, J., Häfeli, U. O., and Bach, H. (2017). Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15, 1–20.
Song, U., and Kim, J. (2020). Zinc oxide nanoparticles: a potential micronutrient fertilizer for horticultural crops with little toxicity. Horticult. Environ. Biotechnol. 61, 625–631.
Soni, N., and Sonam, P. (2014). Green nanoparticles for mosquito control. Sci. World J. 2014, 1–6.
Sowani, H., Mohite, P., Munot, H., Shouche, Y., Bapat, T., Kumar, A. R., et al. (2016). Green synthesis of gold and silver nanoparticles by an actinomycete Gordonia amicalis HS-11: mechanistic aspects and biological application. Process Biochem. 51, 374–383.
Sriram, M. I., Kalishwaralal, K., Barathmanikanth, S., and Gurunathani, S. (2012). Size-based cytotoxicity of silver nanoparticles in bovine retinal endothelial cells. Nanosci. Methods 1, 56–77.
Stepanov, A. L., Nuzhdin, V. I., Valeev, V. F., and Kreibig, U. (2011). “Optical properties of metal nanoparticles,” in Proceedings of the ICONO 2010: International Conference on Coherent and Nonlinear Optics , (Bellingham, WA: SPIE), 543–552.
Su, S. S., and Chang, I. (2018). “Review of production routes of nanomaterials,” in Commercialization of nanotechnologies–a case study approach , eds D. Brabazon, E. Pellicer, F. Zivic, J. Sort, M. D. Baró, N. Grujovic, K.-L. Choy (Berlin: Springer), 15–29.
Sugihartono, I., Dianisya, D., and Isnaeni, I. (2018). “Crystal structure analyses of ZnO nanoparticles growth by simple wet chemical method,” in Proceedings of the IOP Conference Series: Materials Science and Engineering , (Bristol: IOP Publishing), 012077.
Sun, T., Zhang, Y. S., Pang, B., Hyun, D. C., Yang, M., and Xia, Y. J. A. C. I. E. (2014). Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Engl. 53, 12320–12364.
Tavakoli, A. H., Maram, P. S., Widgeon, S. J., Rufner, J., Van Benthem, K., Ushakov, S., et al. (2013). Amorphous alumina nanoparticles: structure, surface energy, and thermodynamic phase stability. J. Phys. Chem. C 117, 17123–17130. doi: 10.1021/jp405820g
Theron, J., Eugene Cloete, T., and De Kwaadsteniet, M. (2010). Current molecular and emerging nanobiotechnology approaches for the detection of microbial pathogens. Crit. Rev. Microbiol. 36, 318–339. doi: 10.3109/1040841X.2010.489892
Thomas, R., Janardhanan, A., Varghese, R. T., Soniya, E., Mathew, J., and Radhakrishnan, E. (2014). Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp. Braz. J. Microbiol. 45, 1221–1227. doi: 10.1590/s1517-83822014000400012
Titus, D., Samuel, E. J. J., and Roopan, S. M. (2019). “Nanoparticle characterization techniques,” in Green synthesis, characterization and applications of nanoparticles , eds A. Shukla and S. Iravani (Amsterdam: Elsevier), 303–319. doi: 10.1016/B978-0-08-102579-6.00012-5
Tran, V., and Wen, X. (2014). “Rapid prototyping technologies for tissue regeneration,” in Rapid prototyping of biomaterials , Ed. R. Narayan (Sawston: Woodhead Publishing), 97–155. doi: 10.1533/9780857097217.97
Triana, M. A., Hsiang, E.-L., Zhang, C., Dong, Y., and Wu, S.-T. (2022). Luminescent nanomaterials for energy-efficient display and healthcare. ACS Energy Lett. 7, 1001–1020.
Uzair, B., Liaqat, A., Iqbal, H., Menaa, B., Razzaq, A., Thiripuranathar, G., et al. (2020). Green and cost-effective synthesis of metallic nanoparticles by algae: Safe methods for translational medicine. Bioengineering 7:129. doi: 10.3390/bioengineering7040129
Van Thai, P., Abe, S., Kosugi, K., Saito, N., Takahashi, K., Sasaki, T., et al. (2019). Size/shape control of gold nanoparticles synthesized by alternating current glow discharge over liquid: The role of pH. Mater. Res. Expr. 6:095074.
Velusamy, P., Das, J., Pachaiappan, R., Vaseeharan, B., and Pandian, K. (2015). Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum ( Azadirachta indica L.). Indus. Crops Products 66, 103–109.
Wang, P., Menzies, N. W., Lombi, E., Sekine, R., Blamey, F. P. C., Hernandez-Soriano, M. C., et al. (2015). Silver sulfide nanoparticles (Ag2S-NPs) are taken up by plants and are phytotoxic. Nanotoxicology 9, 1041–1049. doi: 10.3109/17435390.2014.999139
Wang, Z., Li, H., Tang, F., Ma, J., and Zhou, X. (2018). A facile approach for the preparation of nano-size zinc oxide in water/glycerol with extremely concentrated zinc sources. Nanosc. Res. Lett. 13, 1–9. doi: 10.1186/s11671-018-2616-0
Wiesendanger, R., and Güntherodt, H.-J. (2013). Scanning tunneling microscopy III: theory of STM and related scanning probe methods. Berlin: Springer Science & Business Media.
Xia, Y., Xiao, Z., Dou, X., Huang, H., Lu, X., Yan, R., et al. (2013). Green and facile fabrication of hollow porous MnO/C microspheres from microalgaes for lithium-ion batteries. ACS Nano 7, 7083–7092. doi: 10.1021/nn4023894
Yadav, R., Dwivedi, S., Kumar, S., and Chaudhury, A. (2010). Trends and perspectives of biosensors for food and environmental virology. Food Environ. Virol. 2, 53–63.
Yadav, T. P., Yadav, R. M., and Singh, D. P. (2012). Mechanical milling: a top down approach for the synthesis of nanomaterials and nanocomposites. Nanosci. Nanotechnol. 2, 22–48.
Yang, W., Wang, L., Mettenbrink, E. M., Deangelis, P. L., and Wilhelm, S. (2021). Nanoparticle toxicology. Annu. Rev. Pharmacol. Toxicol. 61, 269–289.
Ye, Q., Chen, W., Huang, H., Tang, Y., Wang, W., Meng, F., et al. (2020). Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 104, 5213–5227. doi: 10.1007/s00253-020-10600-4
Yuan, P., Ding, X., Yang, Y. Y., and Xu, Q. H. (2018). Metal nanoparticles for diagnosis and therapy of bacterial infection. Adv. Healthc. Mater. 7:1701392.
Zahra, Z., Habib, Z., Chung, S., and Badshah, M. A. (2020). Exposure route of TiO2 NPs from industrial applications to wastewater treatment and their impacts on the agro-environment. Nanomaterials 10:1469. doi: 10.3390/nano10081469
Zan, G., and Wu, Q. (2016). Biomimetic and bioinspired synthesis of nanomaterials/nanostructures. Adv. Mater. 28, 2099–2147.
Zhang, X., Yan, S., Tyagi, R., and Surampalli, R. (2011). Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 82, 489–494.
Zheng, Z., Zhang, X., Carbo, D., Clark, C., Nathan, C.-A., and Lvov, Y. (2010). Sonication-assisted synthesis of polyelectrolyte-coated curcumin nanoparticles. Langmuir 26, 7679–7681. doi: 10.1021/la101246a
Zhou, C., Wang, Y., Du, L., Yao, H., Wang, J., and Luo, G. (2016). Precipitation preparation of high surface area and porous nanosized ZnO by continuous gas-based impinging streams in unconfined space. Indus. Eng. Chem. Res. 55, 11943–11949.
Zhou, M., Wei, Z., Qiao, H., Zhu, L., Yang, H., and Xia, T. (2009). Particle size and pore structure characterization of silver nanoparticles prepared by confined arc plasma. J. Nanomater. 2009:968058.
Zhuang, J., and Gentry, R. W. (2011). “Environmental application and risks of nanotechnology: a balanced view,” in Biotechnology and Nanotechnology Risk Assessment: Minding and Managing the Potential Threats around Us , eds S. Ripp and T. Henry (Washington, DC: ACS Publications), 41–67. doi: 10.3390/ijerph16234848
Zielińska, A., Carreiró, F., Oliveira, A. M., Neves, A., Pires, B., Venkatesh, D. N., et al. (2020). Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules 25:3731. doi: 10.3390/molecules25163731
Keywords : green synthesis, nanoparticles, nanotechnology, biological synthesis, microbial nanotechnology, bionanotechnology
Citation: Altammar KA (2023) A review on nanoparticles: characteristics, synthesis, applications, and challenges. Front. Microbiol. 14:1155622. doi: 10.3389/fmicb.2023.1155622
Received: 31 January 2023; Accepted: 21 March 2023; Published: 17 April 2023.
Reviewed by:
Copyright © 2023 Altammar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khadijah A. Altammar, [email protected] ; orcid.org/0000-0002-8691-086X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Significant enhancement in local thermal conductivity of erythritol at interface with nanoparticles due to their interaction
- Research Article
- Published: 16 May 2024
Cite this article

- Ning Cao 1 ,
- Yanhui Feng 1 ,
- Kening Yan 1 ,
- Zihan Liu 1 ,
- Jiawei Wan 2 &
- Lin Qiu 1
21 Accesses
Explore all metrics
When nano-fillers are used to enhance the thermal conductivity of organic phase change materials (PCMs), the naturally formed interface is considered to hinder thermal transport of the composite PCMs. However, the effect of the interface on the thermal properties of surrounding PCM has not been fully studied. In this paper, three composite PCMs (Ery@SiC, Ery@SiO 2 and Ery@Si 3 N 4 ) were prepared by melt-blending method. The local thermal conductivity and reduced Young’s modulus ( E ⋆ ) of the erythritol at the interface and far away from the interface in the composite PCMs were simultaneously measured by scanning thermal microscopy (SThM). The results revealed significant enhancement in local thermal conductivity of erythritol at the interface and its obvious positive correlation with E ⋆ . For different composite PCMs, molecular dynamics (MD) simulations suggested that the increase in intrinsic thermal conductivity and E ⋆ of erythritol is attributed to the increase in interaction energy between erythritol and nanoparticles, as more erythritol phonon vibrations transform from localized mode to delocalized mode and erythritol has a higher density at the interface. These findings will provide new ideas for the design of PCM for energy storage.

This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Effect of Graphite Particle Size on the Thermal Properties of EG/Erythritol CPCM
Synthesis and characterization of novel heat resistant, superparamagnetic poly(ether-imide) nanocomposites containing xanthene: representing a strategy for improving thermal stability of magnetic polymer-based nanocomposites.

Combined effect of poly(ethylene glycol) and boron nitride nanosheets on the crystallization behavior and thermal properties of poly(lactic acid)
Wang, L. G.; Liu, H.; Zhuang, J. H.; Wang, D. S. Small-scale big science: From nano-to atomically dispersed catalytic materials. Small Sci. 2022 , 2 , 2200036.
Article CAS Google Scholar
Su, R. Y.; Chen, J. Y.; Zhang, X. Q.; Wang, W. Q.; Li, Y.; He, R. J.; Fang, D. N. 3D-printed micro/nano-scaled mechanical metamaterials: Fundamentals, technologies, progress, applications, and challenges. Small 2023 , 19 , 2206391.
Lien, D. H.; Retamal, J. R. D.; Ke, J. J.; Kang, C. F.; He, J. H. Surface effects in metal oxide-based nanodevices. Nanoscale 2015 , 7 , 19874–19884.
Article CAS PubMed Google Scholar
Shen, J.; Wang, D. S. How to select heterogeneous CO 2 reduction electrocatalyst. Nano Res. Energy 2024 , 3 , e9120096.
Article Google Scholar
Neudeck, S.; Mazilkin, A.; Reitz, C.; Hartmann, P.; Janek, J.; Brezesinski, T. Effect of low-temperature Al 2 O 3 ALD coating on Ni-rich layered oxide composite cathode on the long-term cycling performance of lithium-ion batteries. Sci. Rep. 2019 , 9 , 5328.
Article PubMed PubMed Central Google Scholar
Reji Kumar, R.; Samykano, M.; Pandey, A. K.; Kadirgama, K.; Tyagi, V. V. Phase change materials and nano-enhanced phase change materials for thermal energy storage in photovoltaic thermal systems: A futuristic approach and its technical challenges. Renewable Sustainable Energy Rev. 2020 , 133 , 110341.
Li, T. X.; Wu, M. Q.; Wu, S.; Xiang, S. Z.; Xu, J. X.; Chao, J. W.; Yan, T. S.; Deng, T.; Wang, R. Z. Highly conductive phase change composites enabled by vertically-aligned reticulated graphite nanoplatelets for high-temperature solar photo/electro-thermal energy conversion, harvesting and storage. Nano Energy 2021 , 89 , 106338.
Gan, T.; Wang, D. S. Atomically dispersed materials: Ideal catalysts in atomic era. Nano Res. 2023 , 17 , 18–38.
Gao, Y.; Liu, B. Z.; Wang, D. S. Microenvironment engineering of single/dual-atom catalysts for electrocatalytic application. Adv. Mater. 2023 , 35 , 2209654.
Han, A. L.; Sun, W. M.; Wan, X.; Cai, D. D.; Wang, X. J.; Li, F.; Shui, J. L.; Wang, D. S. Construction of Co 4 atomic clusters to enable Fe-N 4 motifs with highly active and durable oxygen reduction performance. Angew. Chem., Int. Ed. 2023 , 62 , e202303185.
Chen, Z. P.; Xu, C.; Ma, C. Q.; Ren, W. C.; Cheng, H. M. Lightweight and flexible graphene foam composites for high-performance electromagnetic interference shielding. Adv. Mater. 2013 , 25 , 1296–1300.
Zhao, Y. W.; Zhang, Y. J.; Liu, A. R.; Wei, Z. Z.; Liu, S. Q. Construction of three-dimensional hemin-functionalized graphene hydrogel with high mechanical stability and adsorption capacity for enhancing photodegradation of methylene blue. ACS Appl. Mater. Interfaces 2017 , 9 , 4006–4014.
Liu, F.; Chung, S.; Oh, G.; Seo, T. S. Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal. ACS Appl. Mater. Interfaces 2012 , 4 , 922–927.
Feng, X. R.; Zhang, Y. Y.; Yang, Z. L.; Zhao, Z. H.; Zhu, F.; Wei, X. Y.; Chen, L. X.; Liu, J. L.; Feng, Y. H.; Li, C. M. et al. Polyethylene glycol with dual three-dimensional porous carbon nanotube/diamond: A high thermal conductivity of composite PCM. Nanotechnology 2024 , 35 , 095702.
Yan, K. N.; Qiu, L.; Feng, Y. H. Erythritol/expanded graphite form-stable phase change materials with excellent thermophysical properties. J. Energy Storage 2023 , 68 , 107667.
Qiu, L.; Yan, K. N.; Feng, Y. H.; Liu, X. L. Nano additives-enhanced PEG/AlN composites with high cycle stability to improve thermal and heat storage properties. Energy 2023 , 278 , 127794.
Feng, D. L.; Zhao, Z. H.; Zhang, X. X.; Feng, Y. H. Carbon-based nanoadditives induced enhancement of phase change thermal properties of sugar alcohol and interfacial heat transport mechanisms. Compos. Sci. Technol. 2023 , 243 , 110258.
Zheng, X. B.; Yang, J. R.; Li, P.; Jiang, Z. L.; Zhu, P.; Wang, Q. S.; Wu, J. B.; Zhang, E. H.; Sun, W. P.; Dou, S. X. et al. Dual-atom support boosts nickel-catalyzed urea electrooxidation. Angew. Chem., Int. Ed. 2023 , 62 , e202217449.
Lyu, H.; Feng, D. L.; Feng, Y. H.; Zhang, X. X. Enhanced thermal energy storage of sodium nitrate by graphene nanosheets: Experimental study and mechanisms. J. Energy Storage 2022 , 54 , 105294.
Wang, Q. S.; Zheng, X. B.; Wu, J. B.; Wang, Y.; Wang, D. S.; Li, Y. D. Recent progress in thermal conversion of CO 2 via single-atom site catalysis. Small Struct. 2022 , 3 , 2200059.
Li, R. Z.; Zhao, J.; Liu, B. Z.; Wang, D. S. Atomic distance engineering in metal catalysts to regulate catalytic performance. Adv. Mater. 2024 , 36 , 2308653.
Qiu, L.; Wang, X. T.; Su, G. P.; Tang, D. W.; Zheng, X. H.; Zhu, J.; Wang, Z. G.; Norris, P. M.; Bradford, P. D.; Zhu, Y. T. Remarkably enhanced thermal transport based on a flexible horizontally-aligned carbon nanotube array film. Sci. Rep. 2016 , 6 , 21014.
Article CAS PubMed PubMed Central Google Scholar
Qiu, L.; Wang, X. T.; Tang, D. W.; Zheng, X. H.; Norris, P. M.; Wen, D. S.; Zhao, J. N.; Zhang, X. H.; Li, Q. W. Functionalization and densification of inter-bundle interfaces for improvement in electrical and thermal transport of carbon nanotube fibers. Carbon 2016 , 105 , 248–259.
Shen, Y. F.; Zhang, X. R.; Su, J. P.; Lin, L.; Jiang, Z. Y.; Qiu, L.; Wang, S. D.; Wu, B. J.; Pu, C. Y.; Cai, X. Z. et al. Significantly enhancing mechanical and thermal properties of cellulose-based composites by adding small amounts of lysozyme-modified graphene nanoplatelets via forming strong double-cross-linked interface interactions. ACS Appl. Mater. Interfaces 2023 , 15 , 43159–43168.
Zou, H. Y.; Feng, Y. H.; Zhang, X. X.; Ohara, T.; Qiu, L. Enhancing mechanism of CNT-CNT interface by metal nanoparticle and nanowire effect on the inside and outside of CNT. Int. J. Therm. Sci. 2023 , 185 , 108094.
Yan, X. X.; Zhao, H. B.; Feng, Y. H.; Qiu, L.; Lin, L.; Zhang, X. X.; Ohara, T. Excellent heat transfer and phase transformation performance of erythritol/graphene composite phase change materials. Compos. B Eng. 2022 , 228 , 109435.
Qiu, L.; Zhu, N.; Feng, Y. H.; Zhang, X. X.; Wang, X. T. Interfacial thermal transport properties of polyurethane/carbon nanotube hybrid composites. Int. J. Heat Mass Transfer 2020 , 152 , 119565.
Oya, T.; Nomura, T.; Tsubota, M.; Okinaka, N.; Akiyama, T. Thermal conductivity enhancement of erythritol as PCM by using graphite and nickel particles. Appl. Therm. Eng. 2013 , 61 , 825–828.
Vivekananthan, M.; Amirtham, V. A. Characterisation and thermophysical properties of graphene nanoparticles dispersed erythritol PCM for medium temperature thermal energy storage applications. Thermochim. Acta 2019 , 676 , 94–103.
Zhang, Q.; Luo, Z. L.; Guo, Q. L.; Wu, G. H. Preparation and thermal properties of short carbon fibers/erythritol phase change materials. Energy Convers. Manage. 2017 , 136 , 220–228.
Shao, X. F.; Lin, J. C.; Teng, H. R.; Yang, S.; Fan, L. W.; Chiu, J. N.; Yu, Z. T.; Martin, V. Hydroxyl group functionalized graphene oxide nanosheets as additive for improved erythritol latent heat storage performance: A comprehensive evaluation on the benefits and challenges. Sol. Energy Mater. Sol. Cells 2020 , 215 , 110658.
Guo, S. P.; Liu, Q. B.; Zhao, J.; Jin, G.; Wang, X. T.; Lang, Z. M.; He, W. X.; Gong, Z. J. Evaluation and comparison of erythritol-based composites with addition of expanded graphite and carbon nanotubes. Appl. Energy 2017 , 205 , 703–709.
Shen, S. L.; Tan, S. J.; Wu, S.; Guo, C.; Liang, J.; Yang, Q.; Xu, G. Y.; Deng, J. The effects of modified carbon nanotubes on the thermal properties of erythritol as phase change materials. Energy Convers. Manage. 2018 , 157 , 41–48.
Williams, C. C.; Wickramasinghe, H. K. Scanning thermal profiler. Appl. Phys. Lett. 1986 , 49 , 1587–1589.
Soudi, A.; Dawson, R. D.; Gu, Y. Quantitative heat dissipation characteristics in current-carrying GaN nanowires probed by combining scanning thermal microscopy and spatially resolved Raman spectroscopy. ACS Nano 2011 , 5 , 255–262.
Chen, W. C.; Feng, Y. H.; Qiu, L.; Zhang, X. X. Scanning thermal microscopy method for thermal conductivity measurement of a single SiO 2 nanoparticle. Int. J. Heat Mass Transfer 2020 , 154 , 119750.
Menges, F.; Riel, H.; Stemmer, A.; Gotsmann, B. Nanoscale thermometry by scanning thermal microscopy. Rev. Sci. Instrum. 2016 , 87 , 074902.
Article PubMed Google Scholar
Liu, Z. H.; Feng, Y. H.; Qiu, L. Near-field radiation analysis and thermal contact radius determination in the thermal conductivity measurement based on SThM open-loop system. Appl. Phys. Lett. 2022 , 120 , 113506.
Pfreundschuh, M.; Martinez-Martin, D.; Mulvihill, E.; Wegmann, S.; Muller, D. J. Multiparametric high-resolution imaging of native proteins by force-distance curve-based AFM. Nat. Protoc. 2014 , 9 , 1113–1130.
Dokukin, M. E.; Sokolov, I. Quantitative mapping of the elastic modulus of soft materials with HarmoniX and PeakForce QNM AFM modes. Langmuir 2012 , 28 , 16060–16071.
Şahan, N.; Fois, M.; Paksoy, H. Improving thermal conductivity phase change materials—A study of paraffin nanomagnetite composites. Sol. Energy Mater. Sol. Cells 2015 , 137 , 61–67.
Ho, C. J.; Gao, J. Y. Preparation and thermophysical properties of nanoparticle-in-paraffin emulsion as phase change material. Int. Commun. Heat Mass Transfer 2009 , 36 , 467–470.
Yang, Y. Y.; Luo, J.; Song, G. L.; Liu, Y.; Tang, G. Y. The experimental exploration of nano-Si 3 N 4 /paraffin on thermal behavior of phase change materials. Thermochim. Acta 2014 , 597 , 101–106.
Li, Q. Y.; Katakami, K.; Ikuta, T.; Kohno, M.; Zhang, X.; Takahashi, K. Measurement of thermal contact resistance between individual carbon fibers using a laser-flash Raman mapping method. Carbon 2019 , 141 , 92–98.
Li, Q. Y.; Zhang, X. T-type Raman spectroscopy method for determining laser absorption, thermal conductivity and air heat transfer coefficient of micro/nano fibers. Thermochim. Acta 2014 , 581 , 26–31.
Wu, S.; Li, Q.; Ikuta, T.; Morishita, K.; Takahashi, K.; Wang, R. Z.; Li, T. X. Thermal conductivity measurement of an individual millimeter-long expanded graphite ribbon using a variable-length T-type method. Int. J. Heat Mass Transfer 2021 , 171 , 121115.
Li, D. W.; Li, Q. Y.; Takahashi, K. Thermal resistance mapping along a single cup-stacked carbon nanotube with focused electron beam heating. Int. J. Heat Mass Transfer 2022 , 198 , 123418.
Zhang, Y.; Zhu, W. K.; Hui, F.; Lanza, M.; Borca-Tasciuc, T.; Rojo, M. M. A review on principles and applications of scanning thermal microscopy (SThM). Adv. Funct. Mater. 2020 , 30 , 1900892.
Wang, X. J.; Ho, V.; Segalman, R. A.; Cahill, D. G. Thermal conductivity of high-modulus polymer fibers. Macromolecules 2013 , 46 , 4937–4943.
Li, Y. F.; Mehra, N.; Ji, T.; Yang, X. T.; Mu, L. W.; Gu, J. W.; Zhu, J. H. The stiffness-thermal conduction relationship at the composite interface: The effect of particle alignment on the long-range confinement of polymer chains monitored by scanning thermal microscopy. Nanoscale 2018 , 10 , 1695–1703.
Yang, J. R.; Li, W. H.; Xu, K. N.; Tan, S. D.; Wang, D. S.; Li, Y. D. Regulating the tip effect on single-atom and cluster catalysts: Forming reversible oxygen species with high efficiency in chlorine evolution reaction. Angew. Chem., Int. Ed. 2022 , 61 , e202200366.
Li, W. H.; Yang, J. R.; Wang, D. S. Long-range interactions in diatomic catalysts boosting electrocatalysis. Angew. Chem., Int. Ed. 2022 , 61 , e202213318.
Chen, J.; Zhang, G.; Li, B. W. Remarkable reduction of thermal conductivity in silicon nanotubes. Nano Lett. 2010 , 10 , 3978–3983.
Zhang, Z. W.; Hu, S. Q.; Xi, Q.; Nakayama, T.; Volz, S.; Chen, J.; Li, B. W. Tunable phonon nanocapacitor built by carbon schwarzite based host-guest system. Phys. Rev. B 2020 , 101 , 081402.
Liang, T.; Zhou, M.; Zhang, P.; Yuan, P.; Yang, D. G. Multilayer inplane graphene/hexagonal boron nitride heterostructures: Insights into the interfacial thermal transport properties. Int. J. Heat Mass Transfer 2020 , 151 , 119395.
Yang, L. N.; Yang, N.; Li, B. W. Extreme low thermal conductivity in nanoscale 3D Si phononic crystal with spherical pores. Nano Lett. 2014 , 14 , 1734–1738.
Download references
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2023YFF0612804), the National Natural Science Foundation of China (Nos. 52222602, 52236006, and 22293043), Beijing Nova Program (No. 20220484170), the Fundamental Research Funds for the Central Universities (Nos. FRF-TP-22-001C1 and FRF-EYIT-23-05), Foundation of the Youth Innovation Promotion Association of CAS (No. 2020048), and IPE Project for Frontier Basic Research (No. QYJC-2023-08).
Author information
Authors and affiliations.
School of Energy and Environmental Engineering, University of Science and Technology Beijing, Beijing, 100083, China
Ning Cao, Yanhui Feng, Kening Yan, Zihan Liu & Lin Qiu
State Key Laboratory of Biochemical Engineering, Key Laboratory of Biopharmaceutical Preparation and Delivery, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, 100190, China
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Yanhui Feng , Jiawei Wan or Lin Qiu .
Electronic Supplementary Material
12274_2024_6690_moesm1_esm.pdf, rights and permissions.
Reprints and permissions
About this article
Cao, N., Feng, Y., Yan, K. et al. Significant enhancement in local thermal conductivity of erythritol at interface with nanoparticles due to their interaction. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6690-6
Download citation
Received : 26 March 2024
Revised : 07 April 2024
Accepted : 07 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1007/s12274-024-6690-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- scanning thermal microscopy
- phase change materials
- thermal conductivity
- reduced Young’s modulus
- interaction energy
- phonon participation ratio
- Find a journal
- Publish with us
- Track your research
Plant Virus Treatment Shows Promise in Fighting Metastatic Cancers in Mice
- Liezel Labios - [email protected]
Published Date
Topics covered:.
- Cancer Immunotherapy
- Plant Virus Nanoparticles
- Cancer Metastasis
Share This:
Article content.
An experimental treatment made from a plant virus is effective at protecting against a broad range of metastatic cancers in mice, shows a new study from the University of California San Diego.
The treatment, composed of nanoparticles fashioned from the cowpea mosaic virus—a virus that infects black-eyed pea plants—showed remarkable success in improving survival rates and suppressing the growth of metastatic tumors across various cancer models, including colon, ovarian, melanoma and breast cancer. Similar outcomes were also observed when the treatment was administered to mice whose tumors were surgically removed.
The findings were published recently in Advanced Science .
The new study builds upon previous research by the lab of Nicole Steinmetz, a professor of nanoengineering, director of the Center for Nano-ImmunoEngineering and co-director of the Center for Engineering in Cancer, all at UC San Diego. Steinmetz and colleagues have been using cowpea mosaic virus nanoparticles to trigger the immune system to fight cancer and prevent it from spreading and recurring. In early studies, the approach involved injecting the plant virus nanoparticles directly into tumors to stimulate an immune response. Even though the virus is non-infectious in mammals, the body’s immune cells still recognize it as foreign, triggering a robust immune reaction against the existing tumor, as well as any future tumors.
Now, Steinmetz and her team show that the plant virus nanoparticles do not need to be injected directly into tumors to be effective. Administering the nanoparticles systemically improved survival rates and inhibited metastasis across various cancer types.
“Here, we do not treat established tumors or metastatic disease—we prevent them from forming. We are providing a systemic treatment to wake up the body’s immune system to eliminate the disease before metastases even form and settle,” said Steinmetz.
To make the nanoparticles, the researchers grew black-eyed pea plants in the lab and infected them with cowpea mosaic virus. Millions of copies of the virus were grown and harvested in the form of ball-shaped nanoparticles, which required no further modification before use in experiments. “Nature’s powerful nanoparticles, as produced in black-eyed pea plants,” said Steinmetz.
The researchers tested the efficacy of the treatment in mouse models of colon, ovarian, melanoma and breast cancers. Mice injected with cowpea mosaic virus nanoparticles—and then challenged with metastatic tumors a week later—exhibited improved survival rates and reduced tumor growth compared to untreated mice. Even when challenged with new tumors a month later, treated mice exhibited similar outcomes.
The researchers are particularly excited about the treatment’s effectiveness post-surgery. In another set of experiments, administering the nanoparticles after surgical removal of tumors resulted in improved survival rates and decreased tumor regrowth in mice.
“Even if you perform surgery to remove the tumors, no surgery is perfect and there is outgrowth of metastasis if no additional treatment is provided,” said Steinmetz. “Here, we use our plant virus nanoparticles after surgery to boost the immune system to reject any residual disease and prevent circulating tumor cells from metastatic seeding. We found that it works really, really well!”
The goal is to gear up for clinical trials. As the research progresses, the team will be conducting safety studies and exploring the treatment’s efficacy in pet animals with cancer. Future studies will also focus on understanding the mechanisms underlying the immune-boosting properties of cowpea mosaic virus nanoparticles.
Paper: “ Systemic Administration of Cowpea Mosaic Virus Demonstrates Broad Protection Against Metastatic Cancers .” Co-authors include Young Hun Chung, Zhongchao Zhao, Eunkyeong Jung, Anthony O. Omole, Hanyang Wang and Lucas Sutorus, all at UC San Diego.
This work was supported in part by the National Institutes of Health (R01-CA224605, R01-CA274640, R01-CA253615) and the Shaughnessy Family Fund for Nano-ImmunoEngineering at UC San Diego.
Disclosure: Nicole Steinmetz is a co-founder of, has equity in, and has a financial interest in Mosaic ImmunoEnginering Inc. Steinmetz is a co-founder of, and serves as manager of Pokometz Scientific LLC, under which she is a paid consultant to Mosaic ImmunoEngineering Inc., Flagship Labs 95 Inc., and Arana Biosciences Inc. The other authors declare no potential conflict of interest.
You May Also Like
The climate games, strategic research plan refresh looks to evolve what uc san diego does best, franklin antonio hall sparks research collaborations, metabolism of autism reveals developmental origins, stay in the know.
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.
You have been successfully subscribed to the UC San Diego Today Newsletter.
Campus & Community
Arts & culture, visual storytelling.
- Media Resources & Contacts
Signup to get the latest UC San Diego newsletters delivered to your inbox.
Award-winning publication highlighting the distinction, prestige and global impact of UC San Diego.
Popular Searches: Covid-19 Ukraine Campus & Community Arts & Culture Voices
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 15 May 2024
A large-scale machine learning analysis of inorganic nanoparticles in preclinical cancer research
- Bárbara B. Mendes ORCID: orcid.org/0000-0001-8630-1119 1 na1 ,
- Zilu Zhang ORCID: orcid.org/0009-0000-2180-5957 2 na1 ,
- João Conniot 1 ,
- Diana P. Sousa ORCID: orcid.org/0000-0003-3474-5417 1 ,
- João M. J. M. Ravasco 1 ,
- Lauren A. Onweller ORCID: orcid.org/0009-0004-0865-4495 2 ,
- Andżelika Lorenc ORCID: orcid.org/0000-0002-1474-7864 3 , 4 ,
- Tiago Rodrigues ORCID: orcid.org/0000-0002-1581-5654 3 ,
- Daniel Reker ORCID: orcid.org/0000-0003-4789-7380 2 , 5 &
- João Conde ORCID: orcid.org/0000-0001-8422-6792 1
Nature Nanotechnology ( 2024 ) Cite this article
1007 Accesses
14 Altmetric
Metrics details
- Nanoparticles
- Nanotechnology in cancer
Owing to their distinct physical and chemical properties, inorganic nanoparticles (NPs) have shown promising results in preclinical cancer therapy, but designing and engineering them for effective therapeutic purposes remains a challenge. Although a comprehensive database of inorganic NP research is not currently available, it is crucial for developing effective cancer therapies. In this context, machine learning (ML) has emerged as a transformative tool, but its adaptation to nanomedicine is hindered by inexistent or small datasets. Here we assembled a large database of inorganic NPs, comprising experimental datasets from 745 preclinical studies in cancer nanomedicine. Using descriptive statistics and explainable ML models we mined this database to gain knowledge of inorganic NP design patterns and inform future NP research for cancer treatment. Our analyses suggest that NP shape and therapy type are prominent features in determining in vivo efficacy, measured as a percentage of tumour reduction. Moreover, our database provides a large-scale open-access resource for discriminative ML that the broader nanotechnology community can utilize. Our work blueprints data mining for translational cancer research and offers evidence for standardizing NP reporting to accelerate and de-risk inorganic NP-based drug delivery, which may help to improve patient outcomes in clinical settings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
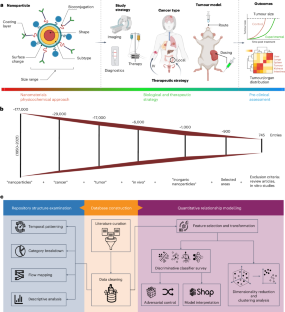
Similar content being viewed by others

Computationally guided high-throughput design of self-assembling drug nanoparticles
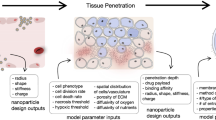
In silico modelling of cancer nanomedicine, across scales and transport barriers
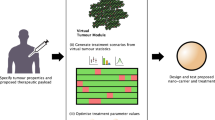
Evolutionary computational platform for the automatic discovery of nanocarriers for cancer treatment
Data availability.
The full database is publicly available via GitHub at https://github.com/RekerLab/NanoAnalysis . Source data are provided with this paper.
Code availability
The code to create our machine learning models and perform the analysis described here is publicly available via GitHub at https://github.com/RekerLab/NanoAnalysis .
Mendes, B. B., Sousa, D. P., Conniot, J. & Conde, J. Nanomedicine-based strategies to target and modulate the tumor microenvironment. Trends Cancer 7 , 847–862 (2021).
Article CAS PubMed Google Scholar
Bobo, D., Robinson, K. J., Islam, J., Thurecht, K. J. & Corrie, S. R. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm. Res. 33 , 2373–2387 (2016).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 4 , e10143 (2019).
Article PubMed PubMed Central Google Scholar
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic: an update post COVID-19 vaccines. Bioeng. Transl. Med. 6 , e10246 (2021).
Article CAS PubMed PubMed Central Google Scholar
Mendes, B. B. et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2 , 24 (2022).
van der Meel, R. et al. Smart cancer nanomedicine. Nat. Nanotechnol. 14 , 1007–1017 (2019).
Janjua, T. I., Cao, Y., Yu, C. & Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 6 , 1072–1074 (2021).
Das, C. G. A., Kumar, V. G., Dhas, T. S., Karthick, V. & Kumar, C. M. V. Nanomaterials in anticancer applications and their mechanism of action - a review. Nanomedicine 47 , 102613 (2023).
Gavas, S., Quazi, S. & Karpiński, T. M. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res. Lett. 16 , 173 (2021).
Faria, M., Björnmalm, M., Crampin, E. J. & Caruso, F. A few clarifications on MIRIBEL. Nat. Nanotechnol. 15 , 2–3 (2020).
Faria, M. et al. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 13 , 777–785 (2018).
Lorenc, A. et al. Machine learning for next-generation nanotechnology in healthcare. Matter 4 , 3078–3080 (2021).
Article CAS Google Scholar
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20 , 101–124 (2021).
Boehnke, N. et al. Massively parallel pooled screening reveals genomic determinants of nanoparticle delivery. Science 377 , eabm5551 (2023).
Article Google Scholar
Brockow, K. et al. Experience with polyethylene glycol allergy-guided risk management for COVID-19 vaccine anaphylaxis. Allergy 77 , 2200–2210 (2022).
Sellaturay, P., Nasser, S., Islam, S., Gurugama, P. & Ewan, P. W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin. Exp. Allergy 51 , 861–863 (2021).
Stone, C. A. Jr. et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J. Allergy Clin. Immunol. Pract. 7 , 1533–1540.e8 (2019).
Article PubMed Google Scholar
Chenthamara, D. et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 23 , 20 (2019).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 , 209–249 (2021).
Global Burden of Disease 2019 Cancer Collaboration. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 8 , 420–444 (2022).
Alvarez, E. M. et al. The global burden of adolescent and young adult cancer in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Oncol. 23 , 27–52 (2022).
Chen, Y., Chen, H. & Shi, J. In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv. Mater. 25 , 3144–3176 (2013).
Iscaro, A., Howard, F. N. & Muthana, M. Nanoparticles: properties and applications in cancer immunotherapy. Curr. Pharm. Des. 25 , 1962–1979 (2019).
Zhou, H. et al. Biodegradable inorganic nanoparticles for cancer theranostics: insights into the degradation behavior. Bioconjug. Chem. 31 , 315–331 (2020).
Zhang, Y. et al. Prolonged local in vivo delivery of stimuli-responsive nanogels that rapidly release doxorubicin in triple-negative breast cancer cells. Adv. Healthc. Mater. 9 , 1901101 (2020).
Conde, J., Oliva, N., Zhang, Y. & Artzi, N. Local triple-combination therapy results in tumour regression and prevents recurrence in a colon cancer model. Nat. Mater. 15 , 1128–1138 (2016).
Kwong, B., Gai, S. A., Elkhader, J., Wittrup, K. D. & Irvine, D. J. Localized immunotherapy via liposome-anchored anti-CD137 + IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer Res. 73 , 1547–1558 (2013).
Li, W. et al. Hyaluronic acid ion-pairing nanoparticles for targeted tumor therapy. J. Control. Release 225 , 170–182 (2016).
Lei, C. et al. Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. J. Am. Chem. Soc. 132 , 6906–6907 (2010).
Fransen, M. F., van der Sluis, T. C., Ossendorp, F., Arens, R. & Melief, C. J. M. Controlled local delivery of CTLA-4 blocking antibody induces CD8 + T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin. Cancer Res. 19 , 5381–5389 (2013).
Ishihara, J. et al. Matrix-binding checkpoint immunotherapies enhance antitumor efficacy and reduce adverse events. Sci. Transl. Med. 9 , eaan0401 (2017).
Errington, T. M., Denis, A., Perfito, N., Iorns, E. & Nosek, B. A. Challenges for assessing replicability in preclinical cancer biology. eLife 10 , e67995 (2021).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1 , 16014 (2016).
Cheng, Y.-H., He, C., Riviere, J. E., Monteiro-Riviere, N. A. & Lin, Z. Meta-analysis of nanoparticle delivery to tumors using a physiologically based pharmacokinetic modeling and simulation approach. ACS Nano 14 , 3075–3095 (2020).
Zhong, R. et al. Hydrogels for RNA delivery. Nat. Mater. https://doi.org/10.1038/s41563-023-01472-w (2023).
Lasagna-Reeves, C. et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 393 , 649–655 (2010).
Hatakeyama, H., Akita, H. & Harashima, H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv. Drug Deliv. Rev. 63 , 152–160 (2011).
Harris, J. M., Martin, N. E. & Modi, M. Pegylation. Clin. Pharmacokinet. 40 , 539–551 (2001).
Suk, J. S., Xu, Q., Kim, N., Hanes, J. & Ensign, L. M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 99 , 28–51 (2016).
Zhang, M. et al. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm. Sin. B 11 , 2265–2285 (2021).
Nguyen, L. N. M. et al. The exit of nanoparticles from solid tumours. Nat. Mater. 22 , 1261–1272 (2023).
Setyawati, M. I. et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE–cadherin. Nat. Commun. 4 , 1673 (2013).
Shamay, Y. et al. Quantitative self-assembly prediction yields targeted nanomedicines. Nat. Mater. 17 , 361–368 (2018).
Reker, D. et al. Computationally guided high-throughput design of self-assembling drug nanoparticles. Nat. Nanotechnol. 16 , 725–733 (2021).
Bannigan, P. et al. Machine learning models to accelerate the design of polymeric long-acting injectables. Nat. Commun. 14 , 35 (2023).
Lundberg, S. M. et al. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2 , 56–67 (2020).
Caballero, D. et al. Precision biomaterials in cancer theranostics and modelling. Biomaterials 280 , 121299 (2022).
Zhao, Y. et al. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 7 , 4131 (2017).
Kolhar, P. et al. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc. Natl Acad. Sci. USA 110 , 10753–10758 (2013).
Zhang, M., Kim, H. S., Jin, T. & Moon, W. K. Near-infrared photothermal therapy using EGFR-targeted gold nanoparticles increases autophagic cell death in breast cancer. J. Photochem. Photobiol. B 170 , 58–64 (2017).
Jo, Y. et al. Chemoresistance of cancer cells: requirements of tumor microenvironment-mimicking in vitro models in anti-cancer drug development. Theranostics 8 , 5259–5275 (2018).
Guo, B. et al. Molecular engineering of conjugated polymers for biocompatible organic nanoparticles with highly efficient photoacoustic and photothermal performance in cancer theranostics. ACS Nano 11 , 10124–10134 (2017).
Li, Z. et al. Small gold nanorods laden macrophages for enhanced tumor coverage in photothermal therapy. Biomaterials 74 , 144–154 (2016).
Das, P., Delost, M. D., Qureshi, M. H., Smith, D. T. & Njardarson, J. T. A survey of the structures of US FDA approved combination drugs. J. Med. Chem. 62 , 4265–4311 (2019).
Fernandes Neto, J. M. et al. Multiple low dose therapy as an effective strategy to treat EGFR inhibitor-resistant NSCLC tumours. Nat. Commun. 11 , 3157 (2020).
Kim, M. H. et al. The effect of VEGF on the myogenic differentiation of adipose tissue derived stem cells within thermosensitive hydrogel matrices. Biomaterials 31 , 1213–1218 (2010).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12 , 2825–2830 (2011).
Google Scholar
Chen, T. & Guestrin, C. XGBoost: a scalable tree boosting system. in Proc. of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 785–794 (Association for Computing Machinery, 2016).
Lundberg, S. M. & Lee, S.-I. A unified approach to interpreting model predictions. in Advances in Neural Information Processing Systems (eds Guyon, I. et al.) Vol. 30 (Curran Associates, Inc., 2017).
Download references
Acknowledgements
This project received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (ERC-StG-2019-848325), the Duke Science and Technology Initiative and the National Institutes of Health NIGMS grant R35GM151255. We acknowledge Fundação para a Ciência e a Tecnologia (FCT) for financial support in the framework of the PhD grant 2020.06638.BD (D.P.S.), the Duke Department of Biomedical Engineering for support through a BME Fellowship (Z.Z.), the National Science Foundation (NSF) for support through the Graduate Research Fellowship DGE2129754 (L.A.O.) and the ERASMUS+ programme (A.L.).
Author information
These authors contributed equally: Bárbara B. Mendes, Zilu Zhang.
Authors and Affiliations
ToxOmics, NOVA Medical School, Faculdade de Ciências Médicas (NMS|FCM), Universidade NOVA de Lisboa, Lisbon, Portugal
Bárbara B. Mendes, João Conniot, Diana P. Sousa, João M. J. M. Ravasco & João Conde
Department of Biomedical Engineering, Duke University, Durham, NC, USA
Zilu Zhang, Lauren A. Onweller & Daniel Reker
Instituto de Investigação do Medicamento (iMed), Faculdade de Farmácia, Universidade de Lisboa, Lisbon, Portugal
Andżelika Lorenc & Tiago Rodrigues
Department of Biopharmacy, Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Toruń, Bydgoszcz, Poland
Andżelika Lorenc
Duke Cancer Institute, Duke University School of Medicine, Durham, NC, USA
Daniel Reker
You can also search for this author in PubMed Google Scholar
Contributions
J. Conde conceived the idea and concept of the study. D.R. conceived the ML platform. T.R. conceived the data curation. B.B.M., J. Conniot, D.P.S., J.M.J.M.R. and J. Conde collected all of the data from the published manuscripts, organized the dataset and calculated the correlations. Z.Z., L.A.O. and A.L. conducted the data analysis, text mining and designed, implemented and evaluated the ML models. J. Conde, D.R. and T.R provided guidance and supervised the work. All authors contributed to the writing and editing of the paper, and all authors approved the final version of the paper.
Corresponding authors
Correspondence to Tiago Rodrigues , Daniel Reker or João Conde .
Ethics declarations
Competing interests.
J. Conde and T.R. are co-founders and shareholders of TargTex SA Targeted Therapeutics for Glioblastoma Multiforme. J. Conde is a member of the Global Burden of Disease (GBD) consortium from the Institute for Health Metrics and Evaluation (IHME), University of Washington, USA, and member of the Scientific Advisory Board of Vector Bioscience, Cambridge. T.R. acts as a consultant to the pharmaceutical, biotechnology and technology industry and is a full member of the Acceleration Consortium, University of Toronto. D.R. acts as a consultant to the pharmaceutical and biotechnology industry, as a scientific mentor for Start2 and serves on the scientific advisory board of Areteia Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information.
Nature Nanotechnology thanks Natalie Boehnke, Karolina Jagiełło and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
Supplementary Results and Discussion, Figs. 1– 9, Tables 1–8 and Refs. 1–11.
Source data
Source data fig. 2.
Alluvial plot source data for Fig. 2c.
Source Data Fig. 4
Raw data for Fig. 4d, alluvial plot source data for Fig. 4e and statistical source data for Fig. 4f.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Mendes, B.B., Zhang, Z., Conniot, J. et al. A large-scale machine learning analysis of inorganic nanoparticles in preclinical cancer research. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-024-01673-7
Download citation
Received : 03 May 2023
Accepted : 10 April 2024
Published : 15 May 2024
DOI : https://doi.org/10.1038/s41565-024-01673-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Nanotechnology is a known field of research since last century. Since "nanotechnology" was presented by Nobel laureate Richard P. Feynman during his well famous 1959 lecture "There's Plenty of Room at the Bottom" (Feynman, 1960), there have been made various revolutionary developments in the field of nanotechnology.Nanotechnology produced materials of various types at nanoscale level.
2.2. Discovery of C, Ag, Zn, Cu, and Au nanoparticles. Carbon NPs were found in 1991, and Iijima and Ichihashi announced the single-wall carbon nanotube synthesis with a diameter of 1 nanometer in 1993 (Chen et al., 2021).Carbon nanotubes (CNTs), also known as Bucky tubes, are a kind of nanomaterial made up of a two-dimensional hexagonal lattice of carbon atoms.
Nanoparticles articles from across Nature Portfolio. Nanoparticles are particles that exist on a nanometre scale (i.e., below 100 nm in at least one dimension). They can possess physical ...
A large-scale machine learning analysis of inorganic nanoparticles in preclinical cancer research. This analysis leverages a large-scale literature review, text mining, statistics and machine ...
Nanoparticles for inducing Gaucher disease-like damage in cancer cells. Increased nutrient uptake of cancer cells offers a possible therapeutic target. Here, the authors designed a nanoparticle to ...
Journal of Nanoparticle Research is a peer-reviewed journal that delves into concepts, properties, phenomena, and processes of structures at the nanoscale. Covered topics include synthesis, assembly, transport, reactivity, and stability of nanoscale structures. Features applications, structures, and devices with novel functions via precursor ...
Nanoparticle chemistry is a relatively young branch of chemical research. Even 30 years ago, these words would have sounded puzzling to many scientists despite the fact that nanoparticles, primarily in the form of dust and smoke, have always existed in nature. Nanoparticles were utilized in construction materials, pigments, and stained glass ...
Importance and meaning. Nanoparticles are small particles ranging from 1 to 100 nm (nm) in size [].They are used in a wide range of applications and can be grouped into four types: 1) inorganic-based nanoparticles, 2) carbon-based nanoparticles, 3) organic/polymer nanoparticles, and 4) composite-based nanoparticles [].Inorganic-based nanoparticles are made up of different metal and metal oxides.
Nanotechnology is one of the most promising key enabling technologies of the 21st century. The field of nanotechnology was foretold in Richard Feynman's famous 1959 lecture "There's Plenty of Room at the Bottom", and the term was formally defined in 1974 by Norio Taniguchi. Thus, the field is now approaching 50 years of research and application. It is a continuously expanding area of ...
Nanoparticle asymmetry shapes an immune response. The chirality, or handedness, of nanoparticles is shown to be a key factor in determining how well such particles engage with the immune system ...
2.3. Nanoparticles and Human Health. NPs can enter the human body in various ways: (i) through the lungs, where they can quickly travel through the bloodstream to crucial organs, including crossing the blood-brain-barrier (BBB), and (ii) through the digestive tract or skin. The material's biocompatibility is vital.
Nanotechnology refers to an emerging field of science that. includes synthesis and development of vario us nanomaterials. Nanoparticles can be defined as objects ranging in size from 1-. 100 nm ...
Nanoparticles from diesel and engine exhaust: In cosmopolitan cities and town, the main source of atmospheric micro- and nanoparticles is automobile exhaust . Amongst the types of automobile exhaust, diesel engines release 20-130 nm sized particles whereas gasoline engines release 20-60 nm sized particles [ 89 - 90 ].
Interest in nanomaterials and especially nanoparticles has exploded in the past decades primarily due to their novel or enhanced physical and chemical properties compared to bulk material. These extraordinary properties have created a multitude of innovative applications in the fields of medicine and pharma, electronics, agriculture, chemical catalysis, food industry, and many others. More ...
Walter Mendes de Azevedo. Brief Report 18 March 2024 Article: 59. 1. 2. …. 150. Next. Journal of Nanoparticle Research is a peer-reviewed journal that delves into concepts, properties, phenomena, and processes of structures at the ...
The exit of nanoparticles from solid tumours. Nanoparticle retention inside tumours has been associated with lymphatic vessel collapse. It is now shown that nanoparticles exit from solid tumours ...
The applications of copper (Cu) and Cu-based nanoparticles, which are based on the earth-abundant and inexpensive copper metal, have generated a great deal of interest in recent years, especially in the field of catalysis. The possible modification of the chemical and physical properties of these nanoparticles using different synthetic strategies and conditions and/or via postsynthetic ...
In addition, related research has focused on nanoparticle-mediated therapy for some other diseases, such as neurodegenerative, infectious, autoimmune, etc. diseases. ... Despite many research articles on the development of novel therapeutic nanoparticles published for AD and PD, only few approaches have been reported for other neurodegenerative ...
Research explores ways to mitigate the environmental toxicity of ubiquitous silver nanoparticles. Credit: Nanomaterials (2024). DOI: 10.3390/nano14080654. Silver has long been used to thwart the ...
Nanoparticles (NPs) can improve the performance and efficiency of energy storage systems used in defense systems, such as batteries or fuel cells ( Morsi et al., 2022 ). In batteries, nanoparticles can be used as a cathode material to increase the battery's energy density, rate capability, and cycling stability.
Under light or thermal stimulus, the nanoparticles switch between their high- and low-spin states, in which they have different volumes. This generates a reversible strain over the MoS 2 layer and ...
This work was supported by the National Key R&D Program of China (No. 2023YFF0612804), the National Natural Science Foundation of China (Nos. 52222602, 52236006, and 22293043), Beijing Nova Program (No. 20220484170), the Fundamental Research Funds for the Central Universities (Nos. FRF-TP-22-001C1 and FRF-EYIT-23-05), Foundation of the Youth Innovation Promotion Association of CAS (No. 2020048 ...
Nature Nanotechnology offers a unique mix of news and reviews alongside top-quality research papers. Published monthly, in print and online, the journal reflects the entire spectrum of ...
An experimental treatment made from a plant virus is effective at protecting against a broad range of metastatic cancers in mice, shows a new study from the University of California San Diego. The treatment, composed of nanoparticles fashioned from the cowpea mosaic virus—a virus that infects black-eyed pea plants—showed remarkable success in improving survival rates and suppressing the ...
Nanoparticle (NP) research for cancer diagnosis and therapy is advancing in medicine, as shown by the increasing number of clinical trials 1,2 and approved products. Breakthroughs in encapsulation ...