An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts


The GABAergic Deficit Hypothesis of Major Depressive Disorder
Bernhard luscher.
1 Departments of Biology, Pennsylvania State University, University Park, PA 16802
2 Departments of Biochemistry & Molecular Biology, Pennsylvania State University, University Park, PA 16802
3 Department of Psychiatry, Pennsylvania State University, College of Medicine, Hershey, PA 17033
4 Center for Molecular Investigation of Neurological Disorders, Pennsylvania State University, University Park, PA 16802
Qiuying Shen
Nadia sahir.
Increasing evidence points to an association between major depressive disorders (MDDs) and diverse types of GABAergic deficits. Here we summarize clinical and preclinical evidence supporting a central and causal role of GABAergic deficits in the etiology of depressive disorders. Studies of depressed patients indicate that MDDs are accompanied by reduced brain concentration of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) as well as alterations in the subunit composition of the principal receptors (GABA A receptors) mediating GABAergic inhibition. In addition, there is abundant evidence that GABA plays a prominent role in the brain control of stress, the most important vulnerability factor in mood disorders. Furthermore, preclinical evidence suggests that currently used antidepressant drugs designed to alter monoaminergic transmission as well as non-pharmacologic therapies may ultimately act to counteract GABAergic deficits. In particular, GABAergic transmission plays an important role in the control of hippocampal neurogenesis and neural maturation, which are now established as cellular substrates of most if not all antidepressant therapies. Lastly, comparatively modest deficits in GABAergic transmission in GABA A -receptor-deficient mice are sufficient to cause behavioral, cognitive, neuroanatomical, and neuroendocrine phenotypes as well as antidepressant drug response characteristics expected of an animal model of MDD. The GABAergic hypothesis of MDD suggests that alterations in GABAergic transmission represent fundamentally important aspects of the etiological sequelae of major depressive disorders that are reversed by monoaminergic antidepressant drug action.
Introduction
Major depressive disorder (MDD) represents a complex neuropsychiatric syndrome with a lifetime prevalence of approximately 17% of the population worldwide 1 . It exhibits high comorbidity with anxiety disorders, with 50–60% of depressed patients reporting a lifetime history of anxiety disorders, and many anxiety disorder patients showing a history of treatment for depression 2 – 9 . Antidepressant drug (AD) treatments currently in use for both anxiety and depressive disorders are designed to target monoaminergic neurotransmission, and they have set the foundation for the so-called catecholamine 10 , 11 and serotonin 12 , 13 hypotheses of affective disorders. Collectively, these hypotheses posit that antidepressants act by increasing the extracellular concentration and function of monoamine transmitters in the forebrain 14 and, by extension, that mood disorders are caused by altered production, release, turnover, or function of monoamine transmitters or altered function of their receptors. There is, however, a growing consensus that altered monoaminergic transmission is insufficient to explain the etiology of depressive disorders 15 and that currently used antidepressants instead are modulating other neurochemical systems that have a more fundamental role in MDD 16 .
A more recent hypothesis suggests that depressive disorders represent stress disorders. It is supported by a large body of epidemiological evidence showing that stress is a major vulnerability factor for mood disorders 17 – 19 . This evidence includes altered HPA axis function in patients 20 , 21 , polymorphisms in the CRH1 (corticotropin releasing hormone 1) receptor gene that are associated with mood disorders 22 , as well as data from rodents showing that central administration of stress-related hormones can produce pathologies reminiscent of MDD, which are reversed by antidepressant drug treatment 23 , 24 . An extension of the stress hypothesis puts forward that depressive disorders are caused by inadequate trophic support of neurons and impaired neural plasticity 25 – 28 . None of the current hypotheses, however, have identified a unified molecular framework that is broadly implicated in the etiology of mood disorders and antidepressant drug mechanisms.
Here we summarize older but underreported and recent or emerging evidence in support of a fourth hypothesis that posits that etiological origins of mood disorders converge on genetic, epigenetic or stress-induced deficits in GABAergic transmission as a principal cause of MDDs, and that the therapeutic effects of currently used monoaminergic antidepressants involve downstream alterations in GABAergic transmission.
GABA and its receptors
Gaba a receptors vs. gaba b receptors.
GABA is the principal neurotransmitter mediating neural inhibition in the brain. GABAergic neurons are present throughout all levels of the neuraxis, represent between 20 and 40% of all neurons depending on brain region, and are known to balance and fine tune excitatory neurotransmission of various neuronal systems including the monoaminergic and cholinergic projections to the forebrain. GABA exerts its effects by activation of two entirely different classes of receptors, the ionotropic GABA A receptors (GABA A Rs) and the metabotropic GABA B Rs. GABA A Rs are known as key control elements of anxiety state based on the potent anxiolytic activity of benzodiazepines (BZs) that act as positive allosteric modulators of a major subset of GABA A Rs. Accumulating evidence described below points to marked alterations in GABA A R signaling in both anxiety and mood disorders. GABA B Rs are members of the G-protein coupled receptor family and they have been recently implicated in affective disorders based on altered anxiety- and depression-related behavioral measures in mice subject to pharmacological and genetic manipulations of these receptors. GABA B (1) and GABA B (2)R KO mice show behavior indicative of increased anxiety combined with an antidepressant phenotype 29 , 30 . Consistent with these genetic studies, positive GABA B R modulators show potential as anxiolytics, whereas antagonists have antidepressant-like effects in animal experiments 29 . However, given the strong evidence for comorbidity of anxiety and depressive disorders, opposing actions of GABA B -directed ligands on anxiety- and depression-related measures are likely to limit the potential of GABA B R-directed therapeutic approaches. Therefore, in this review we will focus on GABA signaling through GABA A Rs, the receptors that mediate the vast majority of GABA function.
Structure of GABA A Rs
Subunit composition.
Structurally, GABA A Rs represent heteropentameric GABA-gated chloride channels that are assembled from subunits encoded by 19 different genes (α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3). Different combination of these subunits give rise to a large number of structurally, functionally and pharmacologically distinct receptor subtypes, of which about 25 have been either definitely or tentatively identified 31 . These can be roughly subdivided into i) postsynaptic and ii) extra- or perisynaptic subtypes, although some neurons also contain GABA A Rs at axon terminals. The postsynaptic GABA A R subtypes include mainly the α1βγ2, α2βγ2, and α3βγ2 receptors whose β subunit remain ill defined; they tend to be concentrated at synapses where they mediate phasic inhibitory synaptic currents in response to synaptically released GABA. The latter consist of α4βδ and α5βγ2 receptors in forebrain and α6βδ in cerebellum. They are located on somatodendritic membrane compartments away from the synaptic cleft and tonically activated by low ambient concentrations of GABA or GABA spilled over from synapses 31 , 32 .
Functional dissociation of different subtypes of BZ-sensitive GABA A Rs
BZs act as positive allosteric modulators of GABA A Rs composed of α1βγ2, α2βγ2, α3βγ2, or α5βγ2 subunits. Using a combined molecular genetic and behavioral pharmacologic strategy these GABA A R subtypes have been assigned to different diazepam-sensitive behaviors based on the specific type of α subunit present 33 , 34 . In particular, it was found that the broadly expressed α1βγ2 receptor subtype mediates sedative, anterograde amnesic, addictive and most of the anticonvulsant effects of diazepam 35 – 38 . In contrast, α2βγ2 receptors control the anxiolytic and anti-hyperalgesic properties 39 , 40 , and α2βγ2, α3βγ2, and α5βγ2 receptors together mediate the myorelaxant effects of diazepam 41 , 42 . The α5βγ2 receptors are further important for normal hippocampus-dependent associative memory functions and for the development of tolerance to the sedative functions of diazepam 42 – 45 . The prevalent distribution of α2βγ2 receptors in the cerebral cortex, hippocampus, and amygdala 46 and the role of this receptor subtype in anxiolysis is consistent with the established role of corticolimbic brain regions in the control of emotional states 47 , 48 . Moreover, the identification of α1βγ2 receptorsin interneurons of the ventral tegmental area (VTA) as substrates for the addictive properties of BZs 37 suggests that functional deficits of these receptors may contribute to anhedonia as seen in GABA A R γ2 subunit-deficient mice 49 (see below). Functional deficits in α1βγ2 receptors can be predicted to increase GABA release by VTA interneurons and to enhance GABAergic inhibition of nearby dopaminergic neurons, and thereby to contribute to anhedonia as a core symptom of major depressive disorder.
BZ insensitive GABA A Rs
In contrast to most postsynaptic γ2-containing GABA A Rs, the extrasynaptic receptor subtypes composed of α4βδ subunits in the forebrain and α6βδ subunits in the cerebellum are insensitive to the GABA-potentiating effects of BZs, and they conduct a prominent tonic form of inhibition. Nevertheless, they exhibit high affinity for the imidazo-BZ Ro15-4513 and flumazenil, as well as the iodinated flumazenil derivative [ 123 I]iomazenil 50 – 52 . These receptors therefore are included along with BZ-sensitive GABA A Rs in autoradiographic and nuclear tomographic measurements using these ligands. The α4βδ receptors are of increasing interest as they are dynamically regulated by stress and other hormonal stimuli implicated in mood disorders.
Brain imaging studies suggest a role for altered GABAergic transmission in anxiety and depressive disorders
Gaba deficits in depression.
The strongest evidence that GABAergic deficits may contribute to depressive disorders is based on reduced GABA levels in plasma 53 , 54 and cerebrospinal fluid 55 or resected cortical tissue 56 of depressed patients. While initial findings were controversial 57 or lacked statistical significance 58 , more recent assessments of GABA deficits in brain using proton magnetic resonance spectroscopy show dramatic reductions of GABA in the occipital cortex 59 , 60 and lower but still significant reductions in the anterior cingulate and dorsomedial/dorsolateral prefrontal cortex 61 , 62 of MDD patients. This neurochemical phenotype is consistent with a selective loss of calbindin positive GABAergic interneurons observed in the dorsal prefrontal cortex of depressed patients 63 . Interestingly, GABA deficits are most pronounced in melancholic and treatment-resistant subtypes of depression (−50%) 56 , 60 , 64 , while reductions in depressed patients not meeting criteria of melancholia 60 and in bipolar patients 65 are less severe (−20%).
GABA A R deficits in anxiety disorders
Reduced abundance of GABA A R binding sites suggests a role for GABAergic deficits in anxiety disorders. Positron Emission Tomography (PET) scanning using the BZ site antagonist 11 C-flumazenil shows global reductions in GABA A R binding sites in patients suffering from panic attacks, with the most robust changes in ventral basal ganglia, orbitofrontal and temporal cortex 66 , which are thought to control the experience of anxiety 67 , 68 . Moreover, while flumazenil has no behavioral effect in healthy people, it precipitates panic attacks during symptom free episodes in panic patients, suggesting unusual inverse agonist properties 69 . Analyses by Single Photon Emission Computed Tomography (SPECT) with a similar ligand ([ 123 I]iomazenil) show widespread reductions in GABA A R binding sites in the superior frontal, temporal, and parietal cortex 70 , left hippocampus and precuneus 71 of panic patients. Similar analyses have revealed GABA A R deficits in the temporal lobe of patients with generalized anxiety disorder 72 and medial prefrontal cortex of patients suffering from posttraumatic stress disorder 73 . Collectively, the data suggest that different anxiety disorders involve GABA A R deficits in different brain regions.
Gene expression changes associated with major depressive disorder suggest altered expression and subunit composition of GABA A Rs
In contrast to anxiety disorders, the density of GABA A R [ 123 I]iomazenil binding sites in brain of depressed subjects is largely unchanged 74 . A notable exception is a single patient suffering from severe treatment-resistant anxious depression with panic attacks linked to a silent point mutation in the GABA A R β1 subunit gene 75 . However, there is abundant evidence for a role of GABA A Rs in major depression based on altered expression of GABA A R subunit transcripts ( Table 1 ). A genome wide screen for changes in transcript levels in the frontopolar cortex [Brodmann area (BA)10] of suicide victims that had suffered from various forms of depressive disorders has revealed reductions in the abundance of α1, α3, α4 and δ subunit mRNAs 76 . Evidence for similarly discoordinated expression of GABA A R subunit transcripts is also available for other brain areas implicated in mood disorders 77 . These studies did not differentiate among changes linked to depression, suicide, or suicide-associated distress, and thus need to be confirmed in a more representative cohort of patients and controls. Interestingly, the reduced expression of the α1 mRNA was associated with increased DNA methylation of transcriptional control regions of the GABRA1 gene and with upregulated expression of the DNA methyltransferase DNMT-3B, suggesting that GABRA1 gene expression is subject to epigenetic control 78 .
Depression related alterations in expression of GABA A R subunit genes.
BA4, motor cortex; BA6, supplementary motor area (medial) and premotor cortex (lateral); BA9/44/46, dorsolateral prefrontal cortex; BA10, frontopolar cortex; BA20, Inferior temporal gyrus; BA21 middle temporal area; BA24, anterior cingulate cortex; BA38 temporopolar area; BA47, ventrolateral prefrontal cortex.
A comparison of postmortem brains of depressed vs. non-depressed suicide victims has revealed increased expression of the α5, γ2, and δ subunit mRNAs in the dorsolateral prefrontal cortex (BA44/46) 79 . This is consistent with an earlier report showing upregulation of β3, γ2 and δ subunit mRNAs in similar brain regions (BA9, 46) of depressed patients who died from more diverse causes 80 . This latter study has further identified selective upregulation of α5 mRNA in the anterior cingulate cortex (BA24), a critical component of the corticolimbic pathway affected in major depression 81 . A comprehensive screen for gene expression changes in 17 cortical and subcortical brain regions from depression-related suicides found that genes that are involved in GABAergic transmission are among the most consistently changed 82 . Among a total of 27 GABAergic probe sets differentially expressed in the frontal cortex or hippocampus no fewer than 19 involve genes that encode GABA A R subunits. GABA A R subunit genes are mostly upregulated in depression-related suicides, perhaps as a compensatory mechanism for low GABA levels associated with depression. Low levels of GABA A R gene expression among suicides that lack a history of depression suggest that elevated expression in depression-related suicides may in fact be depression-specific 82 . These increases in GABA A R subunit mRNAs seem to contradict the aforementioned unaltered levels of GABA A R binding sites 74 in suicide brains. However, altered subunit mRNA levels do not necessarily have to result in changes in GABA A R binding sites, neither of which are representative of functional receptors present at the plasma membrane or at synapses. Discoordinated expression of GABA A R subunits might give rise to functionally distinct GABA A R subtypes that nevertheless bind [ 123 I]iomazenil. Lastly, GABA A Rs are subject to phosphorylation, palmitoylation and ubiquitination, all of which regulate the cell surface expression and accumulation of GABA A Rs at synapses, as well as inhibitory synaptogenesis 83 , 84 . These posttranslational modifications allow for modulation of GABA A R cell surface expression by environmental and physiological cues implicated in mood disorders. Accordingly, mutations in trafficking proteins that regulate the portion of GABA A Rs at synapses affect anxiety and mood-related behavior in both patients 85 and animal models 86 , 87 .
Genetic evidence in support of GABAergic deficits in mood disorders
There is growing evidence that genetic polymorphisms in GABA A R subunit genes are involved in affective disorders. The Wellcome Trust Case Control Consortium has identified a strong association between bipolar disorder (BPD) and polymorphism in the GABRB1 gene coding for the β1 subunit of GABA A Rs 88 . A follow-up study has confirmed this finding and extended it to associations with nucleotide polymorphisms in the GABRA4, GABRB3, GABRA5 and GABRR1 subunit genes 89 . Notably, GABRB1, GABRA4, and GABRR1 are part of the same gene cluster on chromosome 4p12, together with GABRA2, while GABRA5 and GABRB3 are part of a cluster at 15q11-q13, which had previously been implicated in BPD 90 . Associations between nucleotide polymorphisms and BPD further exist for GABRA3 91 and GABRB2 92 , with the latter implicated in alternative splicing of the β2 subunit mRNA 93 . For MDD, genetic associations have been described for GABRA5 94 and the gene cluster encoding GABRA1 95 , 96 , GABRA6 and GABRG2 96 . Although not all studies have found this latter association 97 , this same gene cluster is linked to depression-related behavior also in mice 98 . Finally, there is recent evidence for a male-specific association between non-coding genetic polymorphisms of the GABRD gene and childhood-onset mood disorders 99 . In summary, the data suggest that GABAergic deficit can lead to mood disorders but also demonstrate that genetic polymorphisms at the level of GABA A R subunit genes account for at most a small percentage of mood disorders, and that environmental and remote genetic triggers of GABAergic deficits may be more important.
Modulation of GABA A Rs by stress: a major risk factor of depressive disorders
Effects of early life stress.
Stress represents the most important vulnerability factor for MDD and related neuropsychiatric disorders, both in the developing 100 – 104 and adult nervous system 105 . There is a growing body of preclinical evidence that much of this vulnerability may be due to stress-induced impairment of GABAergic transmission. For example, maternal separation stress of rats during the first postnatal weeks leads to increased neophobia and acoustic startle responses in adulthood, and this phenotype is associated with reduced expression of BZ-sensitive GABA A Rs in the frontal cortex, amygdala, locus coeruleus and the n. tractus solitarius 106 . The level of maternal care measured in the form of pup licking in rodents is positively correlated with GABA A R mRNA expression and inversely related to behavioral stress reactivity in adulthood 107 . Analyses of GABA A R γ2-deficient mice 49 , 108 (further discussed below) suggest that modest reductions in GABA A R function during development are not just correlated with anxiety- and depression-related behavior in adulthood, but that they can be causal.
Effects of stress in adulthood
In addition to early life stress effects on GABA A R expression in the mature brain, there is an extensive literature on stress-induced changes in the expression and function of GABA A Rs in the adult brain. The exact consequences of acute stress on GABA A R expression in rodents appear to depend on the type of stress protocol, sex and brain region(s) analyzed 109 . Most relevant in the context of this review, however, are unpredictable chronic forms of stress that are suitable to model depressive-like symptoms in animal models 110 , 111 . The prevalent effect of chronic stress in the cerebral cortex is reduced abundance and function of GABA A Rs 112 . By contrast, the effects of chronic stress hormone exposure in the hippocampus are uneven and subunit- and layer-specific 113 , 114 . In particular, expression of α4βδ receptors is subject to prominent chronic stress-induced augmentation in granule and pyramidal cell neurons of the hippocampus 115 , 116 . This chronic effect is thought to alter sensitivity of the brain to acute stress-associated increases in neuroactive steroids, as discussed further below.
GABAergic control of HPA axis
Increased secretion of glucocorticoids and aberrant function of the hypothalamic–pituitary–adrenal (HPA) axis are well-replicated findings in a major subset of patients suffering from severe forms of depressive disorders, especially melancholic depression 19 , 21 , 117 – 120 ( Figure 1 ). The paraventricular nucleus (PVN) of the hypothalamus, which is the source of corticotropin-releasing hormone (CRH) that dictates HPA axis responses to stress 121 – 123 , is subject to GABAergic inhibitory control by frontal cortex 122 , 124 and ventral hippocampus 125 . They are activated along with the PVN in response to acute emotional stress 126 and represent major sites of vulnerability to stress 127 – 130 .

The GABAergic deficit hypothesis of MDD presented here suggests that local GABAergic deficits in hippocampus and frontal cortex due to reduced GABA release, uncoordinated GABA A R subunit gene expression or anomalous signaling mechanisms that affect GABA A R accumulation at the plasma membrane lead to local hyperexcitability, which is relayed by projections (In the case of frontal cortex through the BNST 144 ) to the PVN of the hypothalamus. In the hippocampus such local GABAergic deficits may involve loss of parvalbumin positive interneurons 131 , reduced GABAergic synaptic inhibition 130 and reduced maturation and survival of adult-born granule cells 108 , which is sufficient to activate the HPA axis 135 . Cortical deficits in GABAergic inhibition include reduced GABA levels in patients 61 , 62 . In addition, GABAergic deficits may be induced by chronic stress, which down-regulates the expression and function of GABA A Rs in the frontal cortex 112 . Hyperexcitability of the cortex and hippocampus is relayed by projections to the PVN. Local GABAergic inhibition of PVN neurons may be independently compromised by a stress-induced shift in the neural Cl − reversal potential 147 . The ensuing excessive release of CRH from the PVN results in increased release of ACTH from the anterior pituitary, which promotes the release of glucocorticoids, thereby closing a positive feedback loop that amplifies cortical and hippocampal GABAergic deficits. Adrenal neurosteroids normally potentiate GABA-mediated activation of GABA A Rs on dentate gyrus granule cells 168 , 381 . Moreover THDOC upregulates the expression of α4βδ receptors in hippocampal granule cells 115 . However, in CA1 pyramidal cells of the hippocampus the same neurosteroids facilitate GABA-induced desensitization of α4βδ receptors 153 , which increases neural excitability 168 .
In contrast to acute stress, which enhances GABAergic synaptic transmission in the ventral hippocampus 130 , chronic stress causes reductions in GABAergic synaptic currents due to the selective loss of hippocampal parvalbumin-positive interneurons 131 . This effect has been attributed to glucocorticoids acting on a membrane-bound, ill-defined receptor that evokes NO release from hippocampal pyramidal cells 131 . Even modest chronic deficits in GABAergic transmission in GABA A R γ2 +/− mice impair the survival of adult-born hippocampal neurons 108 , an effect that may explain hippocampal volume reductions seen in chronically depressed patients 132 – 134 (see also below). Blocking hippocampal neurogenesis in turn is sufficient to increase HPA axis activity 135 . Thus, projections from the ventral hippocampus via the lateral septum 128 , 136 to the hypothalamus link hippocampal neuropathology to hyperactivity of the HPA axis and aberrant stress reactivity, which may sustain or even amplify hippocampal neuropathology.
Similar to the hippocampus, the dorsomedial and dorsolateral prefrontal and the anterior and subgenual cingulate cortices represent substrates of stress-related psychiatric illness associated with cognitive and affective symptoms of MDD 81 , 129 , 137 – 139 . The deficits in cortical GABA concentrations 61 , 62 and altered expression of GABA A R subunit genes ( Table 1 ) indicate that this phenotype involves reduced GABAergic function. In addition, cortical GABAergic inhibition is impaired by stress-induced signaling pathways, as indicated by drastic CRH-induced, serotonin-mediated desensitization of GABAergic inhibitory synaptic currents recorded from cortical slices 140 . Tracing experiments show that GABAergic neurons of the anterior bed nucleus of the stria terminalis (BNST) serve to relay inhibitory control by the medial prefrontal cortex to the PVN 141 – 144 . Moreover, mice with genetically-induced cortex/hippocampus-restricted GABA A R deficits exhibit chronically elevated HPA axis activity 49 . Thus, local cortical deficits in GABAergic inhibition and correspondingly increased neural excitability lead to increased activity of the PVN, even if the initially causal deficit is limited to extra-hypothalamic circuits (see also below).
In addition to remote inhibition of the hypothalamus by cortical and hippocampal GABAergic circuits, CRH-producing neurons of the PVN themselves are subject to local GABAergic inhibitory control that is regulated by stress 145 . Chronic mild stress of rats results in a marked reduction of the frequency but unaltered amplitude of GABAergic inhibitory synaptic currents recorded from PVN neurons, suggesting presynaptic deficits in GABA release 146 . However, postsynaptic GABAergic function of PVN neurons is also impaired, as indicated by stress-induced down-regulation of the K + -Cl − co-transporter KCC2. The ensuing depolarizing shift of the chloride reversal membrane potential renders GABA inputs ineffective, thereby leading to increased excitability of PVN neurons 147 . Increased CRH release by PVN neurons leads to increased release of adrenocorticotropic hormone (ACTH) by the anterior pituitary gland and systemically elevated basal cortisol levels (corticosterone in rodents) and other stress hormones, which are well-replicated findings in prominent subsets of patients suffering from severe forms of depressive disorders 19 , 117 – 120 , 148 ( Figure 1 ).
GABA A R modulation by neurosteroids
Stress is known to affect GABAergic inhibition at least in part through stress-induced release of endogenous neuroactive steroids that act as allosteric modulators of GABA A Rs. In particular, 3α,5α-tetrahydroprogesterone (THP, also known as [allo]pregnanolone) and 3α,21-dihydroxy-5α-pregnan-20-one (THDOC, [allo]tetrahydrodeoxycorticosterone) are rapidly induced (4 – 20 fold) by stress 149 and known to act as high-affinity modulators of extrasynaptic α4βδ GABA A Rs 150 – 152 . THP either increases (in dentate gyrus granule cells) or reduces (in CA1 pyramidal cells) α4βδ receptor-mediated tonic GABAergic inhibition, due to cell type-specific differences in chloride homeostasis and steroid-induced receptor desensitization, which depends on the direction of the chloride gradient 152 , 153 . Preclinical and clinical data indicate that plasma concentrations of THP and THDOC are reduced and increased, respectively in depressed patients 154 – 157 and normalized by certain ADs (see below), which points to a role for neurosteroid synthesis in the pathology of depressive disorders. While THP is an endogenous metabolite of ovarian/adrenal progesterone and also produced in brain, THDOC is derived exclusively from adrenal sources 149 , 158 , 159 . Normally, α4βδ receptors are readily detectable only in dentate gyrus granule cells, most of the thalamus, striatum, pons, and in the outer layers of cerebral cortex 160 . However, prominent tonic inhibitory currents with a pharmacological profile of δ-containing GABA A Rs in PVN neurons 161 and attenuation of ACTH and corticosterone release by THP and THDOC 162 , 163 indicate that α4βδ receptors also contribute to the inhibitory control of HPA axis activity in the PVN.
The expression of α4βδ receptors is dynamically regulated
In CA1 pyramidal cells the accumulation of these receptors is strongly induced upon progesterone withdrawal 164 – 166 , at puberty 167 , 168 and during pregnancy 166 . In dentate granule cells the abundance of α4βδ receptors is subject to dynamic fluctuations across the ovarian cycle 169 , during pregnancy 166 , 170 , 171 , and induced by stress 115 . Thus, aberrant homeostatic regulation of neurosteroid synthesis together with cell type-specific effects on expression and function of α4βδ receptors is implicated in the etiology of stress-associated mood disorders, premenstrual dysphoric disorder (PMDD) and postpartum depression (PPD) 150 , 151 , 172 , 173 (see below).
Pharmacologic evidence in support of a role of GABAergictransmission in depressive disorders
Antidepressant efficacy of benzodiazepines.
A possible role of GABA A R dysregulation in mood disorders has been controversial in part due to lack of a consensus about whether BZs are therapeutically effective for the treatment of depression 61 . However, the limited use or efficacy of BZs in AD therapies should not be taken as evidence that GABAergic deficits are not involved in the etiology of MDD. Early studies concluded that standard tricyclic antidepressants (TCAs) are overwhelmingly superior to BZs, although the two classes of drugs were initially prescribed for depression almost interchangeably 174 . Indeed, some early studies reported antidepressant efficacy of BZs that was comparable to that of standard antidepressants 175 – 177 , with some studies reporting more rapid therapeutic onset 178 , 179 or greater efficacy of BZs 180 . More recent meta-analyses of clinical data have concluded that antidepressant efficacy of BZs is limited to the triazolo-BZ alprazolam, with classical BZs being ineffective beyond their established role as anxiolytics 181 , 182 . Alprazolam has been rated as equivalent or superior to TCAs with respect to anxiety and sleep indices of depression, equivalent with respect to improving anergia, psychomotor retardation and anhedonia, but inferior in relieving depressed mood 181 , 182 . The most obvious limitations to therapeutic efficacy of BZs are due to rapid development of tolerance, the high risk for developing dependence, the moderate abuse potential, and ultimately the danger of withdrawal symptoms 183 , 184 . At the cellular level, BZs may limit the proliferation of progenitors of adult-born hippocampal neurons, which would limit the effect these drugs can have on immature neurons, which act as a substrate of antidepressant drug action (see below). Nevertheless, BZs are often used in combination with standard antidepressants, even today, both for initial treatment and maintenance therapy 185 , 186 , which suggests beneficial effects. Encouragingly, the sedative hypnotic agent eszopiclone, which acts as a positive allosteric agonist similar to BZs but selectively on α2βγ2 and α3βγ2 subtypes of GABA A Rs, shows significant promise as an antidepressant in patients suffering from insomnia 187 – 189 .
GABAergic mechanisms of monoaminergic antidepressants
With the exception of some BZs mentioned above, currently used antidepressants exclusively target monoamine transmitters. They are designed to block the reuptake of extracellular serotonin (selective serotonin reuptake inhibitors, SSRIs), norepinephrine or, to a lesser extent, dopamine, or they unspecifically inhibit the intracellular degradation of monoamine transmitters. AD-induced increases in extracellular monoamines are thought to result in slow neurochemical, transcriptional, translational, posttranslational, and epigenetic adaptations that underlie therapeutically effective neural plasticity 28 . However, the receptors that mediate the functionally relevant neural adaptations of drug-induced increases in monoamine transmitters and their cellular localization have not been conclusively determined. Indeed, there is evidence that antidepressants may activate G-protein signaling independently of increased monoamine transmitters 190 , 191 . Even so, the antidepressant effects of serotonin in forebrain are thought to involve 5-HT1AR-mediated hyperpolarization of pyramidal cells 192 and 5-HT1B/5-HT2/5-HT3/5-HT4R-mediated excitation of GABAergic interneurons 193 – 197 . In support of this conclusion, the 5-HTR trafficking factor P11/S100A10 interacts with and regulates the cell surface expression and function of 5-HT1B 198 and 5-HT4Rs 199 . Electroconvulsive therapy (ECT) and chronic treatment with imipramine result in upregulation of P11 mRNA and protein selectively in the forebrain 198 . Moreover, P11 is required for normal antidepressant and neurogenic effects of fluoxetine 197 . Importantly, P11 is selectively expressed in several classes of hippocampal GABAergic interneurons but absent in granule cell precursors 197 . Thus, the effects of fluoxetine, imipramine and ECT may have in common that they involve increased excitability of GABAergic interneurons, which, in turn, can be predicted to increase GABAergic activation of hippocampal granule cell precursors 200 , 201 . Whereas GABAergic input to mature neurons is mostly hyperpolarizing, the depolarizing action of GABA on immature granule cells is implicated in the mechanism of monoaminergic AD action (see below).
AD-induced potentiation of GABA release as a mechanism underlying AD effects is congruent with chronic SSRI-mediated increases in cortical GABA concentrations observed in patients 202 and healthy volunteers 203 . However, these reports seem at odds with fluoxetine effects on GABA signaling in the visual cortex of rats 204 . Chronic fluoxetine-induced reductions in cortical GABA concentrations and correspondingly reduced GABAergic inhibition have been shown to reactivate ocular dominance plasticity in the adult brain and to promote the recovery of visual functions in adult amblyopic animals 204 . It remains to be seen whether such effects can be replicated with other antidepressants and whether they extend to brain areas implicated in mood disorders.
Similar to SSRIs, TCAs that increase the extracellular concentration of noradrenalin as well as 5-HT are likely to act in part by modulating GABAergic transmission. Noradrenergic innervation of GABAergic interneurons increases GABAergic transmission in diverse forebrain regions as shown for the frontal 205 , sensorimotor 206 and entorhinal cortices 207 , the CA1 hippocampus 208 and the basolateral amygdala 209 . The selective norepinephrine reuptake inhibitor reboxetine has complex brain region-specific effects on expression of interneuronal glutamic acid decarboxylase 67 (GAD67), the principal enzyme involved in the synthesis of GABA 210 . Immunostaining for GAD67 in brain of medication free depressed suicides is significantly reduced, whereas brain of a different cohort of depressed suicide victims who had been treated with SSRIs or TCAs showed normal levels of GAD67 211 . Collectively, the data suggest that norepinephrine and serotonin reuptake inhibitors have in common that they potentiate GABAergic transmission.
Direct effects of ADs on GABA A Rs
In addition to their principal effects on monoamine transporters and receptors, many if not all antidepressants can directly act on other targets that contribute to therapeutic efficacy, undesirable side effects, or toxicity upon overdose. For example, fluoxetine (1–10 μM) has direct off-target effects at nicotinic acetylcholine 212 , 213 and 5-HT3 receptors 214 – 216 as well as diverse Cl − 217 , voltage-gated Ca 2+ and K + channels 218 – 223 . Importantly, therapeutically relevant concentrations of fluoxetine and its metabolite norfluoxetine act as potent positive allosteric modulators of GABA A Rs in vitro when tested on receptors expressed in heterologous cells 224 and in cultured neurons 225 . This effect may not only contribute to antidepressant efficacy but also explain the unique anticonvulsant properties of fluoxetine in patients 226 .
AD-induced potentiation of GABAergic transmission by neurosteroids
Low concentrations of chronically applied fluoxetine or its active metabolite norfluoxetine and their relatives (i.e. paroxetine, fluvoxamine, sertraline) have been shown to increase the plasma or cerebrospinal fluid (CSF) concentrations of THP 155 – 157 , 227 – 230 . This effect is observed at concentrations fifty-times lower than the concentration that affects 5-HT uptake. Thus, THP appears to contribute to the anxiolytic function of SSRIs 231 . The behavioral effects of THP are independent of an increase in serotonin but are attenuated by bicucullin 232 , which shows that they involve potentiation of GABA A Rs. In vitro experiments with fluoxetine, sertraline, and paroxetine suggest that SSRI-induced increases in THP are due to direct drug effects on enzymes involved in THP synthesis 233 . Hippocampal administration of THP in rats has anxiolytic and antidepressant-like behavioral effects and is associated with increased expression of the γ2 subunit mRNA of GABA A Rs 234 . In addition to genomic effects, THP acts as a potent positive allosteric modulator of mainly α1/4/6βδ subtypes of GABA A Rs 153 , 235 – 239 . These extrasynaptic GABA A Rs are of increasing interest in the context of mood disorders as they are subject to dynamic genomic and hormonal regulation during puberty 167 , 168 , the ovarian cycle 169 , pregnancy 170 , as well as in response to stress 115 , 240 .
The cerebrospinal fluid (CSF) and plasma concentrations of THP are reduced compared to normal controls in drug-free depressed patients 154 – 157 , by social isolation stress in rats 241 , and in the olfactory bulbectomy model of depression of rats 229 . Moreover, SSRIs normalize THP deficits in patients 154 – 156 as well as in bulbectomized rats 150 , 229 , 242 , 243 . Plasma levels of THP are also elevated following partial sleep deprivation 244 , which has antidepressant effects 245 . In contrast to THP, plasma concentrations of THDOC are increased in patients and reduced by fluoxetine 157 . Unlike SSRIs or sleep deprivation, the TCA imipramine 227 , 233 , repetitive transcranial magnetic stimulation 246 and ECT 247 do not affect THP plasma concentrations, suggesting that THP is not universally involved in antidepressant mechanisms. These measurements, however, have yet to be repeated in brain to be conclusive.
In addition to drug therapies, cognitive behavioral therapy 248 and ECT 249 ameliorate cortical GABA deficits in patients. ECT is thought to further enhance GABAergic transmission through an increase in cortical expression of GABA A Rs 250 . Lastly, noradrenergic and serotonergic neurons in the locus coeruleus and raphe nucleus, respectively, are subject to GABAergic control 251 , 252 . In particular, reduced GABAergic inhibition of serotonergic neurons is a developmental risk factor for anxiety and mood disorders, as evidenced by anxiety-and depression-related behavior of mice in which the serotonin transporter was inactivated genetically (KO mice) 253 – 255 or pharmacologically 256 in early life. The collective information on the mechanisms of different antidepressant therapies and their effects on GABA release, neurosteroids synthesis and GABA A R expression and function indicate that enhancing GABAergic transmission lies at the core of both pharmacological and non-pharmacological antidepressant therapies.
GABAergic control of neurogenesis, a target of antidepressant drug treatment
Mechanisms that regulate the production, maturation and survival of adult-born granule cell in the hippocampus (dentate gyrus) have become a focus of research on mood disorders since it was shown in rodents that these processes are enhanced by ADs 257 – 260 and required for many of the AD-induced behavioral responses 259 , 261 – 266 . Conversely, deficits in neurogenesis are a hallmark of genetic and stress-induced animal models of depression 108 , 133 , 267 – 269 and thought to underlie hippocampal atrophy observed in chronically depressed patients 24 , 26 , 27 , 105 , 139 , 270 – 277 . The production of adult-born granule cells is unaffected by serotonin depletion 278 , 279 . Moreover, noradrenaline is dispensable for normal maturation of these neurons, although it is required for normal proliferation of neural precursor cells 278 , 280 . Lastly, we are unaware of any conclusive evidence that monoamine transmitter receptors are expressed on replicating neural progenitors or on immature neurons. The collective evidence suggests that deficits in monoaminergic neurotransmitter systems are unlikely to represent principal culprits of anxiety- and depression-related deficits in hippocampal neurogenesis. By contrast, GABAergic signaling through GABA A Rs has emerged as an essential mechanism that controls proliferation, maturation and survival not only of adult-born neurons in the hippocampus 200 , 201 but also for analogous processes in the postnatal subventricular zone of rodents that replenishes interneurons of the olfactory bulb 281 , 282 and for embryonic neural progenitors that give rise to neurons of the neocortex 283 [for review see 284 , 285 ].
GABAergic mechanisms that control adult hippocampal neurogenesis
GABA A Rs have mainly hyperpolarizing effects on the membrane potential of mature neurons. By contrast, GABA-mediated activation of GABA A Rs is depolarizing and excitatory in proliferating neural progenitors and immature postmitotic neurons 281 , 283 , 285 – 288 ( Figure 2 ). The transition from GABA A R-mediated depolarization to hyperpolarization during the maturation of neurons is triggered by a developmental switch in gene expression of the two Cl − transporters NKCC1 and KCC2, which leads to a gradual shift in the membrane reversal potential of chloride to more negative values. The negative shift of the Cl − reversal potential in turn changes the direction of GABA A R-mediated currents from depolarizing (inward) in neural progenitors and immature neurons to mostly hyperpolarizing (outward) in mature neurons. Importantly, this switch is essential for normal structural and functional maturation and network integration of adult-born granule cells 201 . Short-term enhancement of GABA A R function with barbiturates accelerates the differentiation of proliferating neural progenitor cells and thereby depletes the pool of dividing cells that represents the source of adult born neurons 200 , 281 . In agreement with negative effects of GABAergic inputs on proliferation of new hippocampal neurons, co-administration of fluoxetine with the BZ diazepam negates the effect on proliferation observed with fluoxetine alone 289 . In addition to these effects on proliferating progenitors, GABA-mediated excitation of postmitotic immature neurons results in activation of low threshold T-type Ca 2+ channels 290 , higher threshold L-type Ca 2+ -channels 291 – 294 , and NMDARs 295 . The ensuing increase in intracellular Ca 2+ results in activation of diverse kinases 296 (e.g. CaMKII, PKC, PKA), all of which can phosphorylate Ser133 of the DNA-binding transcription factor CREB (cAMP response element binding protein) and promote the dendritic maturation and survival of these neurons 258 , 297 – 299 ( Figure 2 ).

A. GABA A Rs in immature neurons conduct an inward current (Cl ions moving out of the cell) due to the more positive Cl − reversal potential in these cells. The ensuing membrane depolarization facilitates Ca 2+ entry through V-gated ion channels such as the T-type and L-type voltage gated Ca 2+ channels, and in more mature neurons also NMDARs. The cytoplasmic increase in Ca 2+ results in an increased activity of protein kinases (CaMKII, PKC, PKA, others) that phosphorylate CREB on Ser133. Phosphorylated CREB translocates to the nucleus where it activates a number of target genes including that encoding BDNF. B. Increased production and release of BDNF acts on GABAergic terminals and promotes the release of GABA by TrkB/MAPK-mediated phosphorylation of synapsin and mobilization of GABA-containing vesicles, and by activation of P/Q-type voltage-gated Ca 2+ channels that activate the neurotransmitter release machinery. C, Monoamine transmitters, which are presumed to be elevated in the hippocampus upon AD treatment, act on presynaptic β-adrenergic and 5-HTRs that activate voltage-gated Ca 2+ channels on terminals and soma of GABAergic interneurons. D, Some effects of monoamine transmitters may be mediated by GPCRs on granule cells. However, the expression of these receptors on neural progenitors and immature granule cells has not been documented.
CREB mediates GABAergic control of antidepressant-induced neurogenesis
CREB has a well-established role in learning- and memory-related synaptic plasticity 300 and is involved in hippocampus-mediated AD responses 27 , 301 , 302 and the production, maturation and survival of adult-born hippocampal neurons 258 , 297 , 299 . Consistent with a role of CREB in MDD, CREB expression is down-regulated in brain of depressed (but not schizophrenic) patients studied at autopsy and increased as part of the AD response 303 . All evidence suggests that the effects of ADs on CREB activation and maturation and survival of hippocampal neurons are indirect and downstream of increased GABA signaling via GABA A Rs 299 ( Figure 1 ). Concurrent activation of CREB and increased hippocampal neurogenesis are hallmarks of all currently used antidepressants 257 , 304 , suggesting that their mechanisms of action involve enhancement of GABAergic input to immature granule cells.
Among the transcriptional target genes of CREB, the brain derived neurotrophic factor (BDNF) is of special interest 305 – 307 . BDNF is reduced in serum of depressed 308 , 309 and bipolar patients 310 , 311 and in the dentate gyrus of chronically stressed rats 312 . Conversely, BDNF is induced upon chronic treatment with diverse classes of ADs in the hippocampus of rats 313 , 314 and patients 315 , and it is effective as an antidepressant upon central administration in rodents 316 – 319 . BDNF and its receptor TrkB are essential for normal anxiety-related behavior and for AD behavioral effects in mice 264 , 320 , 321 as well as for normal neural maturation of hippocampal granule cells 322 . Importantly, BDNF is not only a target downstream of excitatory GABAergic transmission but through activation of TrkB receptors on GABAergic terminals serves to promote GABA release 323 , 324 ( Figure 2 ). Thus, BDNF enables a positive feedback loop that upregulates GABAergic signaling, which explains its essential role for normal neural maturation. A related BDNF- and GABA-mediated mechanism protects mature neurons from posttraumatic injury 325 . Currently used AD therapies 314 and ECT all enhance the expression of BDNF 313 , suggesting that these therapies might include enhancement of GABAergic transmission. However, the positive feedback relationship between GABA A R activation, BDNF expression and GABA release may be self-limited to immature neurons (and possibly other neurons with high intracellular Cl − concentrations) as BDNF also promotes the expression of KCC2, which diminishes and eventually eliminates GABAergic depolarization 326 , 327 . Indeed, in contrast to chronic effects of BDNF in immature neurons, acute effects of BDNF at synapses of mature hippocampal pyramidal cells reduce GABAergic transmission 328 – 332 by acting at postsynaptic TrkB receptors that act through PKC and PI-3 kinase-dependent signaling pathways and reduce the surface stability of GABA A Rs 329 , 332 . Moreover, unlike in immature neurons, GABAergic input to adult neurons reduces expression of BDNF 333 .
The neural maturation deficit of dentate gyrus granule cells of BDNF-depleted mice 322 is reminiscent of similar cellular deficits in GABA A R γ2 +/− mice (see below). However, unlike the depressive-like phenotype of γ2 +/− mice detailed further below, mouse lines that are depleted in BDNF or TrkB, do not reliably show behavioral signs of depression, probably reflecting opposing functions of BDNF in the ventral tegmental area (VTA) and nucleus accumbens vs. hippocampus 264 . Moreover, AD-mediated increases in BDNF do not correlate with behavioral effects induced by BDNF administered to different brain regions 334 . Whereas BDNF deficits alone cannot explain the depressive-like phenotypes of GABA A R-deficient mice, a hypomorphic human allele of BDNF (BDNF Val66Met ) is known to interact with environmental stress factors to increase the vulnerability for depression in people 335 – 337 . Preclinical experiments discussed further below suggest that these stress factors involve GABA A R deficits.
The anxiolytic effects of BZs remain intact even when hippocampal neurogenesis has been blocked 263 . This observation and the fact that BZs, unlike ADs, are effective as anxiolytics on acute treatment, indicate that the cellular substrate for anxiolytic effects of BZs is distinct from the one that mediates anxiolytic effects of ADs. Nevertheless, classical BZs are predicted to promote GABA/CREB/BDNF signaling and maturation of adult-born hippocampal neurons. However, drugs that potentiate the function of GABA A Rs do not only promote the maturation of immature neurons, they also seem to accelerate the cell cycle exit of proliferating neural progenitor cells, which delimits the pool of replicating cells and negatively affects neurogenesis 200 , 281 . These putative antagonistic effects of BZs on the total pool of immature dentate gyrus granule cells may explain the limited efficacy of BZs as antidepressants. GABA A R subtype-specific ligands that act selectively on certain GABA A R subtypes might circumvent this limitation. For example, the sedative hypnotic eszopiclone has BZ-like effects mainly on α2βγ2 and α3βγ2 subtypes of GABA A Rs 338 and promotes the survival of adult born hippocampal granule cells in rats without affecting proliferation 339 , 340 . In addition, eszopiclone has promise as a novel non-monoaminergic antidepressant in patients 187 – 189 , 341 .
GABA A R-deficient mice as animal models of depression
Gaba a r γ2 subunit deficient mice and the function of postsynaptic subtypes of gaba a rs, gabaergic deficits cause depressive-like behavioral and cognitive deficits.
The evidence for a role of GABAergic transmission summarized thus far does not prove a causal relationship between GABAergic deficits and depressive disorders. However, corresponding evidence is now available from mice engineered to model depressive disorders. In particular, mice rendered heterozygous for the γ2 subunit (γ2 +/− ) of GABA A Rs have been characterized as an animal model of anxious depression that includes anxious- and depressive-like emotional behaviors in eight different tests 49 , 108 , 342 (for a summary of phenotypes see Table 2 ). The γ2 +/− model is based on a modest functional deficit in postsynaptic GABA A Rs, as evidenced by unaltered GABA A R numbers but reduced punctate immunofluorescent staining representative of postsynaptic GABA A R subtypes and loss of GABA A R BZ binding sites ranging from 6% (amygdala) to 35% (hippocampus) of GABA A Rs, depending on brain region 342 . The magnitude of this deficit is comparable to GABA A R deficits observed in rodents that had been subjected to maternal deprivation stress 106 , 107 , suggesting it is within the pathophysiological range triggered by adverse environments that are implicated in the etiology of mood disorders. The phenotype of γ2 +/− mice includes heightened neophobia and behavioral inhibition to naturally aversive situations 342 , reduced escape attempts under highly stressful conditions 108 , as well as anhedonia-like effects 49 that mimic core symptoms of anxious melancholic depression. Lastly, γ2 +/− mice exhibit selective cognitive deficits such as an attentional bias for threat cues and impaired ambiguous cue discrimination 342 , which are reminiscent of cognitive impairments described in people at risk of or suffering from depression 343 – 348 , and principally attributed to the hippocampus 349 and frontal and cingulate cortex 350 , 351 .
Juxtaposition of phenotypes of Major Depressive Disorder and GABA A R γ2 +/− mice.
GABAergic deficits decrease the survival of adult born hippocampal neurons
Consistent with the hypotheses that depressive disorders represent chronic deficits in neurotrophic support 352 and that GABAergic signaling has trophic function 353 , the γ2 +/− model shows normal proliferation of neural precursor cells but reduced survival of adult-born hippocampal granule cells 108 . The manifestation of this neurogenesis deficit in three different global and conditional γ2-deficient mouse lines is correlated with development of anxious depressive behavior 108 , suggesting that altered neurogenesis and behavioral phenotypes are causally linked.
GABAergic deficits cause HPA axis hyperactivity and increases responsiveness to antidepressant drugs
The neuroendocrine phenotype of γ2 +/− mice includes constitutively elevated serum corticosterone and increased behavioral and endocrine sensitivity to treatment with ADs compared to wild-type mice 49 , which are known characteristics of severely depressed patients 119 , 354 . Selective heterozygous inactivation of the γ2 gene in the developing telencephalic forebrain (including hippocampus and frontal cortex, induced around embryonic day10) is sufficient to induce HPA axis hyperactivity 49 and altered behavior 108 , indicating that the causative GABAergic deficit in these mice is extra-hypothalamic ( Figure 1 ). Glucocorticoids are known to reduce expression of GABA A Rs in the forebrain, particularly in the frontal cortex and ventral hippocampus 114 , 130 , 355 . Moreover, recent evidence indicates that chronic but not acute stress results in loss of parvalbumin positive hippocampal interneurons 131 . Corresponding losses of interneurons in γ2 +/− mice might further enhance GABAergic deficits of γ2 +/− mice and amplify the observed defects in hippocampal neurogenesis. Defects in hippocampal neurogenesis in turn are sufficient to cause HPA axis hyperactivity 135 . Thus, GABA A R deficits in the telencephalon including especially the frontal cortex and hippocampus may be both a cause for, and a consequence of, HPA axis hyperactivity, a feature that may initiate a self-perpetuating feedback loop that amplifies GABAergic deficits, with HPA axis hyperactivity serving as a critical link 49 ( Figure 1 ).
GABAergic deficits cause increased therapeutic efficacy of desipramine compared to fluoxetine
The selective norepinephrine reuptake inhibitor desipramine faithfully reverses both the anxious, depressive-like and anhedonia-like behavioral phenotypes, as well as the elevated serum corticosterone concentrations of γ2 +/− mice 49 . By contrast, fluoxetine shows merely anxiolytic-like activity and fails to normalize depression-related behavior and HPA axis function of γ2 +/− mice. The qualitatively lesser response of γ2 +/− mice to fluoxetine than desipramine is reminiscent of severe subtypes of anxious depressive disorders including melancholic depression, which tend to show greater responsiveness to TCAs than fluoxetine 356 – 363 . Similar to the γ2 +/− model, clinical evidence indicates that elevated basal activity of the HPA axis is linked to poor responsiveness to fluoxetine in patients 356 , 364 , 365 , whereas normalization of HPA axis function by antidepressants is associated with remission from depression 120 , 366 .
The γ2 +/− model shows selective vulnerability to mood disorders during early life
GABAergic transmission acts as key regulator of brain development as indicated by its roles in neurogenesis 201 , neural migration 367 , maturation 108 , and circuit formation 287 , 368 , 369 . In order to delineate the developmental time course and brain regions responsible for the anxious depressive phenotype of γ2 +/− mice, the behavioral and endocrine consequences of γ2 subunit deficits were analyzed in two different conditional mutant strains (Cre-loxP system) 49 , 108 . Mice whose GABA A R deficit is initiated during embryogenesis but limited to the telencephalon were found to replicate the behavioral phenotype and HPA axis hyperactivity of global KO mice, showing that HPA axis hyperactivity can develop independently of primary GABA A R deficits in the hypothalamus 49 . By contrast, delayed inactivation of the γ2 gene during adolescence leads to developmentally delayed HPA axis hyperactivity, which is not accompanied by anxiety or depression-related behaviors 49 , 108 . These data suggest that the anxious depressive-like phenotype of γ2 +/− mice is caused by a developmental GABAergic deficit, whose sequelae include inadequate neurotrophic support in the hippocampus and chronic HPA axis activation. This scenario is consistent with heightened vulnerability to anxiety and mood disorders in people during early life 100 – 104 . In sum, the GABA A R γ2 +/− mouse model includes behavioral, cognitive, cellular, neuroendocrine and developmental dimensions as well as antidepressant drug response characteristics expected of an animal model of melancholic depression and demonstrates that GABA A R deficits can be causative for all these phenotypes.
GABA A R δ subunit-deficient mice and the function of extrasynaptic subtypes of GABA A Rs
Pregnancy and parturition are associated with marked fluctuations in neuroactive steroids, which are linked to changes in mood and anxiety level and known to act mainly through δ subunit-containing, nonsynaptic GABA A R subtypes. Failures of this neuroendocrine system to adapt to rapid changes in ovarian and adrenal hormone level are implicated in postpartum depression (PPD) and postpartum psychosis as evidenced by studies in rodents. Increased brain concentrations of neuroactive steroids during pregnancy of the rat are followed by a sudden drop to control levels within two days of delivery 370 . In rat cortex, late stage pregnancy shows decreased expression of the γ2 and α5 subunits of GABA A Rs and a corresponding reduction in GABA A R function, which rebounds after delivery 371 . In dentate gyrus granule cells and CA1 pyramidal cells, pregnancy of rats is associated with gradually increased and decreased expression of the δ and γ2 subunits of GABA A Rs, respectively, and this effect is normalized within 7 days of delivery 166 . Parturition is further associated with a rapid and transient increase in expression of the α4 subunit in the same cells 166 . The change in GABA A R subunit composition during pregnancy is associated with increased tonic GABAergic inhibition compared to neurons analyzed during estrus and dependent on de novo neurosteroid synthesis 166 .
Pregnancy in mice, unlike in rats, produces a significant downregulation of both the γ2 and δ subunits and corresponding reductions in phasic and tonic GABAergic currents recorded from hippocampal granule cell neurons 170 . Reduced expression of GABA A Rs is thought to compensate for gonadal neurosteroid-mediated increases in GABA A R activity during pregnancy. Postpartum, the expression of GABA A R subunits and the phasic and tonic GABAergic currents recorded from granule cells rebound rapidly to levels found in virgin females. Interestingly, GABA A R δ subunit KO mice, which are unable to adjust expression of δ-containing GABA A Rs show drastic deficits in GABAergic tonic inhibition specifically postpartum, that is associated with anxiety and depression-related behavior as well as abnormal maternal behavior. The pathology of δ subunit KO mice thereby mirrors the symptoms of psychotic PPD 170 .
Dynamic changes in neurosteroid synthesis and GABA A R subunit expression also occur during the estrus cycle, and alterations in these mechanisms are implicated in the etiology of premenstrual dysphoric disorder (PMDD) 169 , 372 . Elevated expression of α4βδ receptors in late diestrus (high-progesterone phase) of the mouse causes increased tonic inhibition of dentate gyrus granule cells along with reduced anxiety 169 . Reduced expression of the δ subunit during estrus is paralleled by upregulation of γ2-containing GABA A Rs, which are comparatively insensitive to neurosteroids. Pharmacological blockade of neurosteroid synthesis from progesterone inhibits cyclic changes in GABA A R subunit expression and neural plasticity while the progesterone receptor antagonist RU486 has no effect, indicating that neurosteroid synthesis rather than nuclear progesterone receptor activation underlies hormone-mediated neural plasticity 115 . Consistent with this interpretation, upregulation of α4βδ receptors and tonic inhibition in hippocampal granule cells can be induced by treatment with THDOC or by acute stress, a condition known to increase neurosteroid levels 115 . Estrus cycle-associated changes in the expression of α4βδ receptors have also been shown in the periaqueductal gray matter of female rats 165 , indicating that neurosteroid–induced plasticity is not limited to the dentate gyrus. In addition to the role of neurosteroids in regulating GABA A R subunit gene expression and as allosteric modulators of α4βδ receptors, neurosteroids have been shown to regulate protein kinase C (PKC)-mediated phosphorylation of GABA A Rs 373 . PKC is known to regulate the cell surface accumulation of GABA A Rs and GABAergic inhibition 374 . In sum, anomalous regulation of α4βδ receptors by neurosteroids at the level of gene expression, channel gating and/or receptor trafficking is implicated in the etiology of PPD and PMDD.
Conclusions, limitations, and outlook
The collective evidence summarized here indicates that reduced concentrations of GABA and altered expression of GABA A Rs are common abnormalities observed in MDDs. GABAergic transmission is vital for the control of stress and impaired by chronic stress, the most important vulnerability factor of MDD. Currently used antidepressants, which are designed to augment monoaminergic transmission, have in common that they ultimately serve to enhance GABAergic transmission. GABAergic excitation of immature neurons in the dentate gyrus has been identified as a key mechanism that provides trophic support and controls the dendritic maturation and survival of neurons, a process that serves as a molecular and cellular substrate of antidepressant drug action. Lastly, comparatively modest deficits in GABAergic transmission are sufficient to cause most of the cellular, behavioral, cognitive and pharmacological sequelae expected of an animal model of major depression. GABAergic transmission is further subject to dynamic regulation by estrus- and pregnancy-associated changes in steroid hormone synthesis and altered expression of extrasynaptic GABA A Rs that may contribute preferentially to female-specific risk factors of mood disorders and explain the increased prevalence of MDD in the female population. The behavioral phenotypes in GABA A R γ2 +/− and δ subunit knockout mice suggest that deficits in both synaptic and nonsynaptic GABAergic transmission can contribute to depressive disorders.
Despite remarkable recent progress we are left with a number of significant gaps in understanding. GABAergic deficits are not unique to MDD but similarly implicated in a number of other neuropsychiatric disorders, especially schizophrenia 375 , 376 . The question arises whether and how GABAergic deficits can help to differentiate between these different disorders. Moreover, the mechanisms that lead to initial GABAergic deficits remain poorly understood and they are so far not explained by mutations or functional polymorphisms in genes intimately involved in GABAergic transmission. We have listed a number of reasons that explain why currently available GABA potentiating drugs are ineffective as antidepressants, yet it remains to be established whether next generation GABAergic drugs that are more selective for GABA A Rs expressed in corticolimbic circuits affected in depression exhibit more convincing efficacy as antidepressants. Furthermore, a number of aspects of major depressive disorders are not know to involve GABAergic deficits. For example, there is increasing preclinical evidence that resilience to stress and stress-induced neuropsychiatric disorders including depression are subject to epigenetic mechanisms 377 , yet there is little evidence for epigenetic regulation of GABAergic transmission. Transcriptional and immunohistochemical alterations in brain of depressed patients suggest links between depressive disorders and inflammation, apoptosis 378 and oligodendrocyte dysfunction 379 , 380 , but none of these have been linked to GABAergic deficits. Future research should address these gaps in understanding and lead the path to improved antidepressant therapies that strive to correct the causal neurochemical imbalances rather than merely the symptoms of depression.
Acknowledgments
We thank Byron Jones, Pam Mitchell and Casey Kilpatrick for critical reading of the manuscript. Research in the Luscher laboratory is supported by grants MH62391, MH60989 and RC1MH089111 from the National Institutes of Mental Health (NIMH), and a grant from the Pennsylvania Department of Health using Tobacco Settlement Funds. The contents of this review are solely the responsibility of the authors and do not necessarily represent the views of the NIMH or the NIH. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.
Conflicts of Interest. The authors declare no conflict of interest
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 26 June 2023
Understanding the mechanism of action and clinical effects of neuroactive steroids and GABAergic compounds in major depressive disorder
- Andrew J. Cutler ORCID: orcid.org/0000-0001-5800-0378 1 ,
- Gregory W. Mattingly ORCID: orcid.org/0000-0002-0234-6860 2 &
- Vladimir Maletic 3
Translational Psychiatry volume 13 , Article number: 228 ( 2023 ) Cite this article
9614 Accesses
11 Citations
48 Altmetric
Metrics details
- Molecular neuroscience
- Neuroscience
The pathophysiology of major depressive disorder (MDD) is thought to result from impaired connectivity between key brain networks. Gamma-aminobutyric acid (GABA) is the key inhibitory neurotransmitter in the brain, working primarily via GABA A receptors, with an important role in virtually all physiologic functions in the brain. Some neuroactive steroids (NASs) are positive allosteric modulators (PAMs) of GABA A receptors and potentiate phasic and tonic inhibitory responses via activation of synaptic and extrasynaptic GABA A receptors, respectively. This review first discusses preclinical and clinical data that support the association of depression with diverse defects in the GABAergic system of neurotransmission. Decreased levels of GABA and NASs have been observed in adults with depression compared with healthy controls, while treatment with antidepressants normalized the altered levels of GABA and NASs. Second, as there has been intense interest in treatment approaches for depression that target dysregulated GABAergic neurotransmission, we discuss NASs approved or currently in clinical development for the treatment of depression. Brexanolone, an intravenous NAS and a GABA A receptor PAM, is approved by the U.S. Food and Drug Administration for the treatment of postpartum depression (PPD) in patients 15 years and older. Other NASs include zuranolone, an investigational oral GABA A receptor PAM, and PH10, which acts on nasal chemosensory receptors; clinical data to date have shown improvement in depressive symptoms with these investigational NASs in adults with MDD or PPD. Finally, the review discusses how NAS GABA A receptor PAMs may potentially address the unmet need for novel and effective treatments with rapid and sustained antidepressant effects in patients with MDD.
Similar content being viewed by others

Modulators of GABAA receptor-mediated inhibition in the treatment of neuropsychiatric disorders: past, present, and future

Novel neurosteroid therapeutics for post-partum depression: perspectives on clinical trials, program development, active research, and future directions
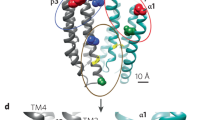
Neurosteroids: mechanistic considerations and clinical prospects
Introduction.
Depression is a common and debilitating mental health disorder that negatively impacts a person’s health and functioning and is a leading cause of disability globally [ 1 ]. According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, text revision, major depressive disorder (MDD) is characterized by ≥2 weeks of at least 5 of the following symptoms (at least 1 of which is depressed mood or anhedonia): depressed mood, anhedonia (loss of interest or pleasure in daily activities), feelings of guilt/low self-esteem, changes in sleep, weight loss or loss of appetite, psychomotor retardation or agitation, fatigue, poor concentration, and suicidal thought that represents a change from previous functioning. The major depressive episode must not be due to another disorder [ 2 ]. The global annual age-standardized prevalence of depressive disorders (MDD and dysthymia) in 2019 was estimated to be 3440.1 per 100,000 individuals [ 3 ]. Based on the 2021 National Survey on Drug Use and Health (NSDUH) in the United States, the annual prevalence of major depressive episodes in adults was 8.3% and that of major depressive episodes with severe impairment was 5.7% [ 4 ].
The etiology of depression has not yet been fully established, but considering the heterogeneity of symptoms, underlying genetics, and treatment responses, it is generally believed that the cause of MDD may be multifactorial. Various genetic and environmental factors (eg, first-degree family members with MDD, adverse childhood experiences, stressful life events) can influence the development of depression [ 2 ]. A genome-wide association study of genetic and health records of 1.2 million individuals from 4 separate data banks identified variations in 178 genes that were linked to MDD [ 5 ]. Stressful environmental signals can be integrated into the genome via epigenetic mechanisms, such as DNA methylation and histone modifications [ 6 , 7 ]. Evidence also shows that modified DNA methylation patterns due to stress can affect brain plasticity and emotion in patients with depression [ 8 ]. Furthermore, brain imaging studies have shown structural and functional changes associated with depression [ 9 , 10 , 11 ]. Structural changes include a loss of glial cells, morphologic changes in neurons, and decreased volume in the cingulate cortex, prefrontal cortex (PFC), hippocampus, and amygdala [ 12 ]. Functional changes in MDD involve abnormal connectivity in the central executive, default mode, and salience networks, the key neuronal networks controlling mood, arousal, behavior, and cognition [ 13 , 14 ]. Elevated activity of the hypothalamic-pituitary-adrenal (HPA) axis, neurotrophic deficit, and neuroinflammation are other potential mechanisms proposed for the development of depression [ 15 , 16 ].
More recent data implicate alterations in the sensorimotor network as being the most consistent factor in depression [ 17 , 18 ]. Evidence also suggests an association of altered connectivity in the default mode network with postpartum depression (PPD) [ 19 ]. These functional networks communicate using several neurotransmitters, including amino acids such as glutamate and gamma-aminobutyric acid (GABA), the primary excitatory and inhibitory neurotransmitters in the brain, respectively, and monoamines such as norepinephrine, dopamine, and serotonin [ 12 , 20 ].
Hypotheses of depression
Several hypotheses exist for the pathophysiology of depression as it relates to altered neurotransmitter levels. The early monoamine hypothesis, which posits that a core pathophysiologic feature of depression is depletion of brain monoamine neurotransmitters (eg, norepinephrine, dopamine, and serotonin), originated from the observation that most standard-of-care antidepressant therapies (ADTs) can increase extracellular concentrations of these neurotransmitters [ 15 , 16 , 21 , 22 ].
The glutamatergic hypothesis of depression suggests an association between elevated glutamate levels and depression [ 16 , 22 ]. This hypothesis is based on preclinical evidence of the antidepressant effects of N-methyl-D-aspartate (NMDA)-receptor antagonists [ 23 ]. Glutamate binds to NMDA receptors, resulting in excitatory neurotransmission [ 24 ]. Elevated glutamate levels lead to overactivation of NMDA receptors and induce calcium ion (Ca 2+ ) influx, which in turn may lead to long-term potentiation and long-term depression [ 25 , 26 ]. However, the evidence for elevated glutamate levels in depression is inconsistent. A postmortem study of adults with MDD reported increased glutamate levels in the frontal cortex of patients with MDD [ 27 ], and a proton magnetic resonance spectroscopy study showed increased glutamate levels in the occipital cortex of patients with MDD [ 28 ]. Conversely, a meta-analysis of proton magnetic resonance spectroscopy studies examining levels of glutamatergic neurometabolites reported significant decreases in the combined glutamine-plus-glutamate level within the medial PFC in patients with depression compared with healthy volunteers but not in the dorsolateral PFC or medial temporal cortex; differences in glutamate levels between the two groups were not significant in any of these areas [ 29 ]. Another meta-analysis showed that glutamate levels were lower within the anterior cingulate cortex of patients with depression compared with healthy volunteers [ 30 ].
The GABAergic deficit hypothesis proposes that defects in GABAergic neural inhibition causally contribute to the common phenotypes of MDD, and, conversely, the efficacy of an ADT may be linked to its ability to restore GABAergic neurotransmission [ 31 ]. It is based on findings of reduced levels of GABA in the plasma, cerebral cortex, and cerebrospinal fluid (CSF), altered expression and subunit composition of GABA A receptors, and reduced levels of neuroactive steroids (NASs) in CSF among individuals with depression [ 22 , 32 ]. The hypothesis is also supported by results from multidisciplinary contemporary approaches that combined large-scale genome-wide association studies, postmortem cytology, and functional and structural imaging studies to clarify the shared origins of otherwise biologically heterogeneous MDD [ 33 , 34 ]. A consistent association was noted between principal neuroimaging findings in individuals with depression and downregulated genetic markers for cortical somatostatin-expressing GABAergic interneurons and astrocytes [ 33 ]. Polygenic somatostatin interneuron markers were most expressed in the subgenual anterior cingulate, medial PFC, anterior insula, and temporal lobes, coinciding with brain areas where imaging studies confirmed cortical thinning and aberrant connectivity in individuals with depression [ 33 ]. The expression of the MDD-associated somatostatin gene marker SST was found to be significantly negatively correlated with structural differences in cortical regions of individuals with MDD relative to healthy controls [ 34 ]. Impaired GABAergic signaling is also thought to be implicated in PPD [ 35 , 36 ] and bipolar disorder [ 37 , 38 ].
This narrative review provides preclinical and clinical data supporting the role of GABAergic signaling in the brain and the GABAergic deficit hypothesis of depression, and how modulation of GABA signaling by GABAergic compounds and NASs could potentially be employed to treat depression. We also examine currently approved and investigational NAS therapies and their hypothesized mechanisms of action in depression, supporting the potential link of science and practice for physicians and clinical researchers. The mechanisms and novel therapies reviewed may impact the approach to rapid treatment of MDD with improved long-term outcomes. Publications were selected from the literature based on their relevance to the covered topics (ie, the role of GABA signaling and the potential role of GABAergic compounds and NASs in MDD) and author experience and preference.
GABAergic signaling and normal brain functioning
The complex interplay between excitatory glutamatergic neurons and inhibitory GABAergic neurons is essential to achieving balanced cortical neural activity [ 39 , 40 ]. The glutamatergic-GABAergic balance is tightly regulated by the biosynthesis, transport, and signaling of the respective neurotransmitters (ie, glutamate and GABA) in the central nervous system (CNS) [ 39 , 40 ]. The biosynthesis of glutamate and GABA are interrelated via the glutamate/GABA-glutamine cycle [ 41 ]. Briefly, glutamatergic neurons release glutamate via synaptic vesicles into the synaptic cleft, where it is taken up by astrocytes and converted to glutamine. Glutamine is transported back to glutamatergic neurons, hydrolyzed to glutamate, and repackaged into synaptic vesicles [ 41 ]. GABAergic neurons release GABA into the synapse, where it is taken up by astrocytes and ultimately converted to glutamine. Glutamine is then transported to GABAergic neurons, where it is converted to glutamate by glutaminase and then to GABA by glutamate decarboxylase [ 42 ].
Physiologic role of GABA and GABAergic neurons
Excitatory glutamatergic and inhibitory GABAergic neurons predominantly communicate through synaptic interactions [ 43 ]. GABA is present primarily in local interneurons, but also in long projection neurons in the PFC, anterior cingulate cortex, amygdala, nucleus accumbens, ventral tegmental area, and the hippocampus—the regions functionally associated with decision-making, cognition, intelligence, memory, sleep, emotions, motivation, and pleasure [ 12 , 44 , 45 , 46 , 47 ]. GABA plays an important role in neuronal proliferation, migration, differentiation, and preliminary circuit-building during brain development [ 44 , 48 ] and is implicated in the development of interstitial neurons in white matter and oligodendrocytes [ 44 ]. GABA also regulates connectivity between the major brain functional networks (eg, default mode and executive control networks) [ 49 ].
GABAergic projection neurons are widely distributed throughout the brain and make dense connections between brain regions involved in mood regulation and reward learning (Fig. 1 ) [ 12 ]; GABAergic interneurons play a vital role in local neural circuitry and activity [ 50 ]. Altogether, GABAergic neurons play an important role in regulating various physiologic brain functions, such as learning and memory, sensorimotor processing, and neuroplasticity [ 51 ]. GABAergic neurons also terminate the innate physiologic stress response by regulating the HPA axis and restoring homeostasis, suggesting the critical role of GABAergic signaling in normal brain function [ 45 ].
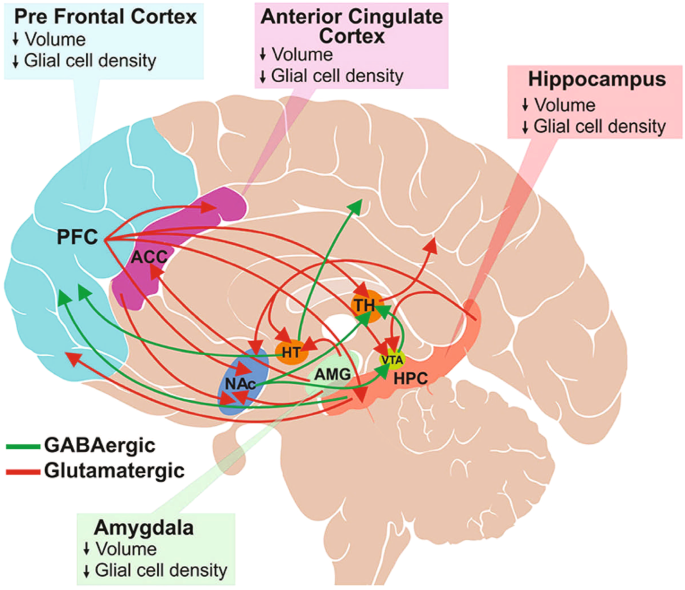
Glutamatergic projections illustrated here include those from the frontal cortex to the anterior cingulate cortex (ACC), thalamus (TH), ventral tegmental area (VTA), hippocampus (HPC) and nucleus accumbens (NAc); from hippocampus to hypothalamus (HT), VTA, NAc and PFC; and from amygdala to HT, ACC and NAc. Major GABAergic projections are from HT to the occipital and parietal cortex, HPC to PFC, and from NAc to TH and VTA. Only a subset of known interconnections is shown here. Depression is associated with reduced brain volume and decreased glial cell density in various brain regions, including ACC, PFC, hippocampus, and amygdala. (Figure is reproduced from Sarawagi et al. 2021 [ 12 ] according to the terms of the Creative Commons Attribution License [CC BY]).
GABA receptors
GABA mediates neural inhibition in the brain by activating the 2 major GABA receptors: (1) GABA A , ionotropic ligand-gated ion channels that signal via direct ligand-mediated opening; and (2) GABA B , metabotropic G protein-coupled receptors that act indirectly via intracellular signaling cascades [ 32 , 52 , 53 ]. GABA B receptor-mediated signaling relies on the activation of G protein signaling pathways to inhibit neurotransmitter release and modulate action potential propagation [ 52 , 54 ]. GABA B receptor expression or activity does not appear to be consistently altered in individuals with depression and has therefore attracted less research interest compared with GABA A receptors in this disease [ 55 ]. However, GABA B receptor activation has been reported to increase membrane trafficking of GABA A receptors in dentate gyrus granule cells, resulting in enhanced GABA A receptor current [ 56 , 57 ].
GABA A receptors are widely distributed in the brain and play an important role in many brain functions [ 58 , 59 ]. In addition to GABA, other endogenous ligands include zinc, NASs, and certain amino acids [ 60 , 61 , 62 ]. GABA A receptors are encoded by 19 subunit genes, six α (α1-α6), three β (β1-β3), three γ (γ1-γ3), three ρ (ρ1-ρ3), and one each of the δ, ε, π, and θ subunits [ 63 ]. GABA A receptors belong to a large heterogeneous class of pentameric chloride channels comprising 2 α, 2 β, and 1 γ, δ, ρ, θ, or ε subunits, with the α1β2γ2 GABA A receptors being the most abundant [ 32 , 53 ]. The complex and heterogenous nature of GABA A receptors results in considerable diversity in their physiology, location, and pharmacologic profile [ 53 ]. Upon GABA binding and activation of the GABA A receptor, chloride ions flow into the cell, leading to rapid membrane hyperpolarization and inhibition of action potentials in the postsynaptic neuron [ 52 , 64 ]. A subclass of GABA A receptors, termed GABA A -ρ (previously GABA C ) receptors, is a group of receptors composed exclusively of ρ subunits, which are typically insensitive to GABA A allosteric modulators (eg, benzodiazepines, barbiturates, and most NASs) [ 65 ]. However, there are some GABA A receptor modulators that can also engage GABA A -ρ receptors, such as pregnanolone, allopregnanolone, and some synthetic NASs [ 65 , 66 , 67 ].
The subunit composition of the GABA A receptor defines its biophysical and pharmacologic properties and whether it localizes to a synaptic or extrasynaptic site. The widely expressed α1–3β1–3γ2 GABA A receptors are predominantly localized to the synapses, while the α4–6β2–3δ GABA A receptors are largely present extrasynaptically [ 53 , 68 , 69 ]. Activation of low-affinity synaptic γ subunit-containing receptors is transient and mediates rapid phasic inhibition, while extrasynaptic δ subunit-containing receptors mediate tonic inhibition through persistent activation by low concentrations of ambient extracellular GABA [ 64 , 70 ]. In neurons with both synaptic and extrasynaptic conductance, the tonic currents may produce a larger net inhibitory effect than do the phasic currents [ 71 ].
Neuroactive steroids
Neuroactive steroids are a class of steroids that are synthesized de novo in neurons and glia of the central and peripheral nervous systems following transport of cholesterol into the mitochondria (Fig. 2 ). Additionally, some circulating sterols (eg, progesterone, dehydroepiandrosterone [DHEA]) can cross the blood-brain barrier to be used as precursor molecules [ 64 , 72 , 73 , 74 , 75 ]. Endogenous NASs are generally categorized as: (1) pregnane-derived (eg, allopregnanolone, allotetrahydrodeoxycorticosterone [allo-THDOC]); (2) androstane-derived (eg, androstanediol, etiocholanolone); or (3) sulfated (eg, pregnenolone sulfate, dehydroepiandrosterone sulfate) [ 64 , 72 ].

There it is metabolized by P450scc into pregnenolone, the precursor of all endogenous NASs. Biosynthetic enzymes are denoted in green; neuroactive steroids and substrates are denoted in red. *Allotetrahydrodeoxycorticosterone is also known as tetrahydrodeoxycorticosterone (same Chemical Abstract Services number). Allo-THDOC allotetrahydrodeoxycorticosterone, DHEAS dehydroepiandrosterone sulfate, DHT 5α-dihydrotestosterone, HSD hydroxysteroid dehydrogenase, NAS neuroactive steroid, P450aro cytochrome P450-aromatase, P450c11β cytochrome P450 11β-hydroxylase, P450c17 cytochrome P450 17α-hydroxylase, P450c21 cytochrome P450 21-hydroxylase, P450scc cytochrome P450 side chain cleavage, PREGS pregnenolone sulfate, SULT sulfotransferase.
Neuroactive steroids regulate neuronal excitability via rapid non-genomic action [ 64 ], primarily through interaction with neuronal membrane receptors and ion channels like the ionotropic GABA A receptors [ 64 , 72 ]. Activity of NASs at neuronal GABA A receptors occurs within minutes, compared with the slow-onset (delayed by hours) and prolonged duration of action of steroid hormones, which act via intracellular steroid hormone receptors [ 76 ]. In general, NASs bind to GABA, NMDA, serotonin, and σ-1 receptors to modulate neurotransmitter signaling [ 72 ]. They modulate excitatory-inhibitory balance and homeostatic mechanisms, thus regulating brain functions that control mood, aggression, cognition, memory, and pain [ 77 ]. Neuroactive steroids can function as positive allosteric modulators (PAMs) of both synaptic and extrasynaptic GABA A receptors to activate and potentiate phasic and tonic currents, respectively [ 64 , 78 , 79 , 80 ], or as negative allosteric modulators (NAMs) to dampen the response to neurotransmitter ligands such as glutamate and GABA [ 81 ]. NAS GABA A receptor NAMs are activation-dependent, non-competitive inhibitors of GABA A receptors and can also inhibit the effects of NAS GABA A receptor PAMs [ 80 ]. NAS GABA A receptor PAMs also regulate neuroplasticity, neuroinflammation, and HPA axis function and may play an important role in neurogenesis [ 36 , 82 , 83 , 84 ]. Allopregnanolone, pregnanolone, and allo-THDOC are among the more potent endogenous NAS PAMs of GABAergic neurotransmission [ 85 ].
GABA A receptor activation by NASs occurs via 2 discrete sites in the α and β subunit transmembrane domains, one at the α-β subunit interface for activation and the other exclusively on α subunits for potentiation of response to NASs [ 62 , 79 ]. Binding of nanomolar concentrations of NAS GABA A receptor PAMs increases the mean open time and decreases the mean closed time of the GABA A receptor chloride channel in the presence of sub-saturating concentrations of GABA, thereby increasing the chloride current through the channel and reducing neuronal excitability [ 64 , 86 ]. In the absence of GABA, micromolar concentrations of PAMs can directly open GABA A receptor chloride channels [ 87 ]. Interestingly, NAS GABA A receptor PAMs have also been shown to increase phosphorylation of certain GABA A receptor subunits, leading to increased cell surface expression of those GABA A receptors [ 88 , 89 , 90 ]
The sensitivity of GABA A receptors to NASs is determined by the receptor subunit composition; at normal extracellular GABA concentrations, extrasynaptic δ subunit-containing GABA A receptors are more sensitive to NAS modulation compared with synaptic γ subunit-containing GABA A receptors [ 91 ], allowing for greater enhancement in GABA A receptor currents [ 92 ]. This preferential interaction of NASs with extrasynaptic δ subunit-containing receptors is secondary to GABA acting as a partial agonist at these receptors [ 92 ]. However, allopregnanolone modulates both γ- and δ subunit-containing GABA A receptors within a similar potency range and may therefore enhance both phasic and tonic currents, respectively [ 93 ].
Dysregulated GABAergic signaling
Major depressive disorder has been linked to dysregulation of the excitatory-inhibitory balance within the brain and the reduced ability to maintain homeostasis in response to internal or external stimuli [ 12 , 45 , 94 , 95 ]. Both preclinical and clinical data support an association of depression with diverse defects in GABAergic neurotransmission. Epigenetic changes of the GABAergic system have been shown to be responsible for adult hippocampus neurogenesis and depression-like behaviors in prenatal-stressed mice [ 96 ]. In addition, alterations in DNA methyltransferase mRNA expression have been observed in the brains of individuals with MDD who died by suicide compared with the brains of non-MDD/suicide individuals, and this change in expression was associated with gene-specific aberrations in DNA methylation in the GABA A receptor α1 subunit promoter region within the frontopolar cortex [ 97 ]. Alterations in the DNA methylation signatures of GABA-related genes have also been reported in other psychiatric disorders, including autism spectrum disorder [ 98 ], schizophrenia [ 99 , 100 ], psychosis with a history of chronic alcohol abuse [ 101 ], and bipolar disorder [ 102 ].
A review of postmortem studies found variable expression of various GABA A receptor subunit mRNAs of suicide victims with depressive disorders and patients with MDD [ 32 , 103 ]. A large human gene expression analysis of cortical and subcortical regions from the brains of depression-related suicides found that the expression levels of genes involved in GABAergic transmission were among the most consistently changed [ 104 ]. Genetic alteration of the γ-subunit of the GABA A receptor disrupted the regulatory response of GABAergic neurons and led to depressive and anxiogenic behaviors in rodents [ 105 , 106 , 107 ]. Genetic studies in mice have shown that deletion of the GABA A receptor γ subunit led to impaired GABAergic signaling and behavioral and cognitive deficits that could be reversed by chronic desipramine or acute ketamine [ 105 , 108 ], while deletion of the α2 subunit led to depressive- or anxiety-like behaviors [ 109 , 110 ]. Mice with decreased GABA A receptor δ subunit expression displayed anxiety-like behavior and maternal neglect postpartum, and administration of the δ subunit selective agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) reduced the abnormal behaviors [ 111 ]. Similarly, administration of SGE-516, a NAS GABA A receptor PAM, rescued these abnormal behaviors in this same model [ 112 ].
GABA levels and GABA A receptor function were found to be diminished in several brain regions in rodent models of acute and chronic stress [ 113 , 114 , 115 , 116 ]. GABA A receptor agonists or GABA A receptor PAMs prevented and reversed rodent behavioral models of depression [ 117 ]; conversely, administration of GABA A receptor antagonists to normal rodents caused behaviors that mimicked these models of depression [ 118 ]. Individuals with depression exhibit reduced functioning of GABAergic interneurons and defects in GABAergic neural inhibition compared with healthy controls [ 28 , 119 , 120 ]. Calbindin-D28K, a calcium-binding and buffering protein critical for preventing neuronal death as well as maintaining calcium homeostasis, is expressed ubiquitously across multiple brain regions that are intimately involved in regulating emotional behaviors, particularly in GABAergic interneurons in the PFC, amygdala, and hippocampus [ 121 ]. A postmortem study showed that the density and size of GABAergic interneurons immunoreactive for calbindin-D28K were significantly decreased in the PFC of individuals with MDD versus those without MDD [ 122 ]. Positron emission tomography imaging showed reduced GABA A receptor binding of [ 11 C]-flumazenil in the limbic parahippocampal temporal gyrus and right lateral superior temporal gyrus of individuals with MDD versus healthy controls, suggesting a decreased number of GABA A receptors and/or reduced affinity to benzodiazepine-site ligands [ 123 ]. GABAergic inhibitory neurotransmission in cerebral cortex, as assessed using transcranial magnetic stimulation, has been shown to be reduced significantly in individuals with MDD [ 124 ].
Individuals with depression, compared with healthy controls, also exhibited diminished GABA levels in the brain, plasma, and CSF [ 38 , 125 , 126 ] that are most pronounced in melancholic and treatment-resistant depression [ 119 , 127 ], and remission from MDD was accompanied by normalization of GABA levels in the brain [ 125 ]. Additionally, severity of anhedonia is inversely correlated with GABA levels in the anterior cingulate cortex as shown in adolescents with MDD [ 128 , 129 ], further supporting the correlation between dysregulated GABAergic neurotransmission and depression. Findings of reduced levels of glutamate decarboxylase in postmortem PFC of individuals with untreated MDD compared with healthy controls provide additional evidence for a link between GABAergic dysfunction and depression [ 130 ]. ADTs that affect monoaminergic neurotransmission may also show downstream effects on GABA- and glutamatergic neurotransmission [ 22 , 131 ]. In animal models, studies showed that selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs) decrease glutamatergic signaling [ 132 , 133 , 134 ]. Treatment with SSRIs or electroconvulsive therapy in individuals with depression has resulted in normalization of decreased plasma GABA levels in the brain and plasma [ 135 , 136 , 137 ]. Due to limited study group sizes, no significant correlation was found between measures of clinical response and the change in cortical GABA concentrations in 2 of these studies [ 136 , 137 ]. However, in a study of inpatients with MDD treated with SSRIs, 70% of responders had increased GABA levels and 64% had decreased glutamate levels [ 135 ].
Dysregulated GABA neurotransmission is also linked to anxiety and insomnia, 2 common comorbidities in individuals with depression. An association between anxiety and GABAergic signaling is supported by the preclinical findings of anxiety behaviors related to chronic inhibition of GABA synthesis [ 138 ] and the disruption of the anxiolytic-like effect of diazepam due to diminished levels of glutamate decarboxylase [ 139 ]. Additionally, review of the therapeutic mechanism of action for different anxiolytics found that these drugs may share a final common pathway involving enhancement of GABAergic neurotransmission [ 131 ]. GABA is also believed to be involved in the regulation of sleep [ 140 ]. Time awake after sleep onset has been found to be inversely correlated with GABA levels in individuals with primary insomnia, although results regarding changes in GABA levels of individuals with primary insomnia versus healthy controls are inconsistent [ 141 , 142 ]. Furthermore, drugs targeting GABA A receptors, such as benzodiazepines and Z -drugs, exhibit sedative and hypnotic effects [ 143 , 144 ].
GABAergic and monoaminergic neurons are interconnected, and, consequently, GABA A receptor deficits can also alter dopaminergic, serotonergic, and noradrenergic activity [ 32 ] (Fig. 3 ). Additionally, inadequate signaling in somatostatin-positive GABAergic interneurons in prefrontal microcircuits (established as one of the key substrates in MDD) can potentially produce attenuated pyramidal neuron output from the PFC and subsequent downstream regulation of threat and danger circuits (amygdala and bed nucleus of the stria terminalis) and sensory and motor processing in the thalamus, mimicking monoamine insufficiency in the brainstem [ 145 , 146 ].
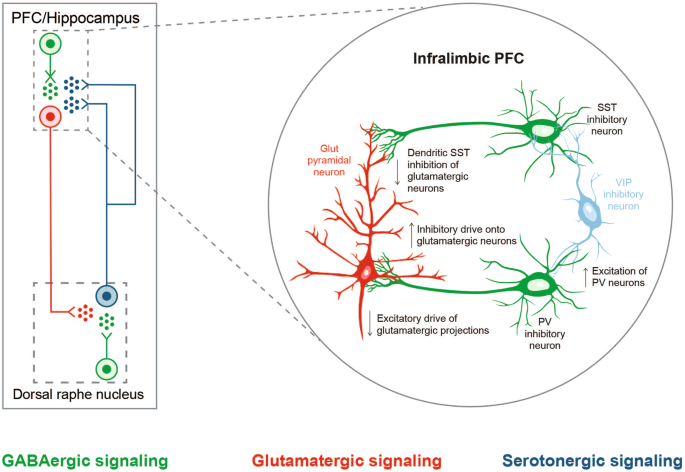
Serotonergic neurons originating in the dorsal raphe nucleus and projecting to the prefrontal cortex (PFC) regulate excitability of GABAergic and glutamatergic neurons, which in turn, modulate the excitability of serotonergic neurons in the dorsal raphe nucleus by the GABA-glutamate balance (left). Chronic stress affects local networks regulating activity within the medial PFC (mPFC), leading to changes in local excitatory-inhibitory balance. In a proposed mechanistic model (right), somatostatin-expressing GABAergic neurons provide reduced dendritic inhibition of glutamatergic pyramidal neurons in the infralimbic mPFC under chronic stress, reducing filtering of information flow into the PFC [ 145 ]. An altered glutamate and GABA neurotransmission might appear as a disturbance in monoamine signaling. (Part of this figure is adapted from McKlveen et al. 2019 [ 145 ], with permission from Elsevier).
Role of neuroactive steroids in depression and other brain disorders
The downregulated biosynthesis of NAS GABA A receptor PAMs has been implicated in various psychiatric disorders (eg, MDD, PPD, premenstrual dysphoric disorder, and posttraumatic stress disorder [PTSD]) [ 31 , 147 ]. Changes in NAS GABA A receptor PAMs synthesis pathways have been linked to the pathologies of neurodegenerative and inflammatory brain diseases (eg, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis) based on postmortem studies [ 148 ], as well as to self-reported pain symptoms (eg, chest pain, muscle soreness) [ 149 ] in humans. Levels of allopregnanolone have been shown to be significantly decreased in individuals with PTSD, a condition that is highly comorbid with MDD [ 150 ].
Reduced levels of allopregnanolone in the CSF and plasma have also been reported in individuals with mood disorders such as MDD, in addition to decreased GABA levels [ 151 , 152 ] and decreased PFC expression of 5α-reductase, the enzyme catalyzing the rate-limiting step in allopregnanolone biosynthesis [ 153 ]. Decreased levels of allopregnanolone in the plasma or serum were also found in individuals with postpartum “blues” or pharmacologically induced panic attacks [ 154 , 155 ], in contrast to the increased level of the 3β isomer of allopregnanolone, which antagonizes GABA A receptor function in panic attacks [ 148 ]. Significant fluctuations in the blood and brain levels of allopregnanolone were shown to be strongly correlated with alterations in function and plasticity of GABA A receptors in rodents [ 156 , 157 ]. The failure to upregulate GABA A receptors in response to the rapid drop in levels of allopregnanolone postpartum is likely involved in the development of PPD [ 35 ].
While acute stressors can lead to increased levels of NAS GABA A receptor PAMs (ie, allopreg-nanolone and allo-THDOC) in animal models [ 36 , 158 ], chronic stress, a major predictor of MDD, was shown to result in altered GABAergic signaling and decreased production of endogenous GABA A receptor PAMs (ie, allopregnanolone) in rodent stress models [ 80 , 159 , 160 ]. Chronic stress-induced reduction in allopregnanolone levels was associated with abnormal behaviors such as aggression, enhanced fear, depressive- or anxiety-like behaviors, and impaired adult hippocampal neurogenesis in animal models [ 161 , 162 , 163 , 164 ].
Selective serotonin reuptake inhibitors such as fluoxetine and norfluoxetine can normalize decreased levels of allopregnanolone in the brain while decreasing behavioral abnormalities associated with mood disorders, as demonstrated in socially isolated mice [ 165 , 166 ]. Studies in individuals with depression also showed that treatment with fluoxetine could increase allopregnanolone levels in the CSF [ 151 , 152 ], and these changes were correlated with improvements in depressive symptoms [ 151 ]. The important role of NAS GABA A receptor PAMs in depression is further supported by the findings that allopregnanolone administration prevented or normalized depressive- or anxiety-like behaviors in a social isolation rodent model [ 163 ].
GABA A receptor positive allosteric modulators
Most GABA A receptor-targeting drugs (ie, barbiturates, benzodiazepines, and NASs) function via allosteric binding to the receptor at sites distinct from the GABA binding sites (Fig. 4 ) [ 58 , 62 , 79 , 91 , 167 , 168 ]. GABA binding sites are located at the α-β subunit interface on both synaptic and extrasynaptic receptors [ 58 ]. Barbiturates, benzodiazepines, and NASs bind the GABA A receptor at allosteric sites and increase the GABA A receptor current by increasing chloride conductance [ 169 ]. The presence of GABA is necessary for benzodiazepine response. Binding of benzodiazepines to the synaptic GABA A receptor locks the receptor into a conformation for which GABA has much higher affinity, thus increasing the frequency of the chloride channel opening, with minimal effect on the duration of bursts [ 170 , 171 ]. Barbiturates, on the other hand, bind in the presence of GABA to both synaptic and extrasynaptic GABA A receptors and increase the duration of chloride channel opening without altering the frequency of bursts [ 169 , 171 ]. Only at high doses can barbiturates directly stimulate GABA A receptors in the absence of GABA [ 172 ].
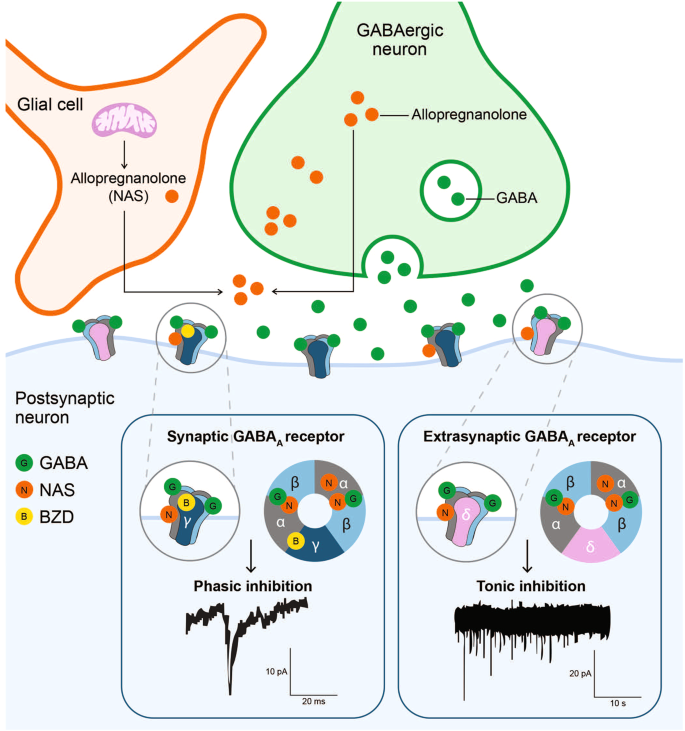
NAS GABA A receptor PAMs, such as allopregnanolone, bind to GABA A receptors at sites distinctive from those for benzodiazepines (BZDs). NAS GABA A receptor PAMs bind to both synaptic γ subunit-containing and extrasynaptic δ subunit-containing GABA A receptors, potentiating phasic and tonic currents, respectively. In contrast, benzodiazepines bind to γ subunit-containing GABA A receptors only and primarily augment phasic inhibition. Extrasynaptic GABA A receptors containing δ subunits are insensitive to benzodiazepines [ 32 , 53 , 62 , 64 , 78 , 79 , 91 , 92 , 167 ].
Barbiturates bind to the α-β subunit interface on synaptic and extrasynaptic receptors and to γ-β subunit interfaces on synaptic receptors [ 58 ]. Barbiturates also non-selectively bind to the entire superfamily of ligand-gated ion channels [ 173 ]. Barbiturates act as antagonists of ionotropic glutamate receptors, such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors, thus inhibiting the bulk of fast excitatory synaptic transmission and glutamate release throughout the CNS [ 173 ]. These actions may account for the antidepressant, anxiolytic, hypnotic, and anticonvulsant activities of barbiturates, but may also account for the physical and psychological addiction potential and high rates of tolerance and dependence associated with this class of drugs. Barbiturates also have a high overdose potential due to a very narrow dosage margin [ 174 ]. Barbiturates were used to treat depression, anxiety, and insomnia in the early part of the 20th century but were generally replaced by benzodiazepines in the 1960s because the many potential drawbacks outweighed their usefulness [ 175 ].
Benzodiazepines are sometimes used to treat specific symptoms that are frequently associated with depression (eg, anxiety and insomnia). However, while a meta-analysis showed that treatment with the benzodiazepine alprazolam led to a higher percentage of individuals with MDD achieving response on the 17-item Hamilton Rating Scale for Depression (HAMD-17; ≥50% reduction in total score) or Clinical Global Impression-Improvement (CGI-I) scale (much improved or very much improved) compared with placebo, other benzodiazepines such as chlordiazepoxide and diazepam did not exhibit clear antidepressant activity [ 176 ]. In addition, chronic use of benzodiazepines more than 2–4 weeks is not recommended as it may result in decreased GABAergic and monoaminergic function, cognitive and psychomotor impairment, and interference with neurogenesis [ 177 , 178 ]. These concerns, in addition to the risk of dependence and abuse [ 179 ] and an overall increase in the risk of attempting or completing suicide [ 180 ] may further limit the potential use of benzodiazepines in the treatment of depression [ 178 ].
Given that benzodiazepines appear to have questionable antidepressant activity, it is speculated that lack of antidepressant activity may be associated with the subunit composition of the GABA A receptors with which benzodiazepines interact. Benzodiazepines bind at the α-γ subunit interface on synaptic GABA A receptors (primarily augmenting phasic inhibition) [ 58 ]. Preclinical data suggest that α1 subunit-containing GABA A receptors play a major role in sedation and addiction [ 181 , 182 ], positive modulation of α2 subunit-containing receptors may have more consistent antidepressant effects [ 109 ], and activation of α3 subunit-containing receptors have pro-depressant actions [ 183 ]. Benzodiazepines show a higher affinity for α1 subunit-containing receptors [ 53 ] and concomitantly activate α2- or α3 subunit-containing receptors [ 184 ], the net effect of which is the promotion of sedation and addiction and a null effect on depression. In addition, benzodiazepines may only enhance phasic inhibitory currents through their binding to synaptic (γ subunit-containing) GABA A receptors [ 185 ]. GABA A receptor PAMs that bind to both synaptic and extrasynaptic GABA A receptors and enhance both phasic and tonic inhibitory currents, respectively, may have greater therapeutic utility than benzodiazepines in treating depression.
The ability of NASs to target both synaptic and extrasynaptic GABA A receptors is especially important in conditions where synaptic GABA A receptors are downregulated, a condition which could lead to benzodiazepine tolerance [ 185 ]. NASs can interact with most GABA A receptors, including the benzodiazepine-insensitive receptors containing α4 and α6 subunits or lacking the γ subunit [ 91 ]. In addition to allosteric modulation of GABA A receptors, NAS GABA A receptor PAMs can also exert metabotropic effects on GABAergic inhibition via activation of the G protein-coupled membrane progesterone receptors (mPRs); mPR-dependent modulation of GABA A receptor phosphorylation results in increased cell surface expression of GABA A receptors and thus a sustained elevation in tonic current [ 90 ], further differentiating them from benzodiazepines, which are associated with a downregulation of GABA A receptors [ 186 ]. The tonic current is resistant to the competitive GABA A receptor antagonist gabazine, confirming that it is not generated from GABA binding to these receptors [ 187 ].
Current treatment options for mdd in clinical practice
The 8 general groups of approved drugs for MDD are: SSRIs, SNRIs, TCAs, tetracyclic antidepressants (TeCAs), MAOIs, atypical and multimodal antidepressants, NMDA receptor antagonists, and GABA A receptor modulators (Table 1 ) [ 188 , 189 , 190 , 191 , 192 , 193 , 194 ].
Standard-of-care ADTs used in the current pharmacologic management of MDD primarily target monoamine neurotransmitter systems. Compared with first-generation ADTs (TCAs and MAOIs), SSRIs and SNRIs have generally been shown to cause relatively fewer adverse effects and therefore appear to be more widely used [ 195 , 196 ]. A meta-analysis of the efficacy and tolerability of SSRIs against TCAs in patients with MDD showed that although TCAs demonstrated similar efficacy with SSRIs (with superior efficacy in hospitalized patients), they are associated with significantly more adverse effects due to their inhibition of cholinergic, α-1 adrenergic, and histaminergic receptors [ 197 ]. TCAs are also more likely to induce toxicity and can be fatal if overdosed [ 198 ]. Although efficacious, MAOIs are not commonly prescribed because of potentially fatal reactions including increased blood pressure, heart attack, stroke, or serotonin syndrome when used together with foods containing high levels of tyramine (eg, aged cheese, spoiled meat, soy sauce) or other drugs [ 199 , 200 ]. Monoaminergic ADTs often require 4 to 6 weeks or longer to take effect [ 201 , 202 , 203 ]. In addition, the STAR*D Study has shown that as many as approximately 50% of patients may not respond adequately [ 203 ]. Relapse rates can be high in patients taking standard-of-care ADTs, especially in those who require multiple treatment steps, as demonstrated in the STAR*D Study among patients with MDD (relapse rates ranged from 40%–70% during a 12-month naturalistic follow-up) [ 202 ].
Novel ADTs with targets that have been implicated in the neurobiology of depression beyond monoamines (eg, glutamate and GABA), are being investigated. For example, while the mechanism of action for the antidepressant effects of ketamine is not fully understood, it is thought that it may block NMDA receptors on GABAergic interneurons, thereby preventing their activation [ 204 ]. Subsequently, downstream disinhibition of glutamatergic neurons causes a glutamate surge. Increased extracellular glutamate initiates activation of postsynaptic AMPA receptors, leading to potentiation of BDNF and mTORC1 synaptogenic signaling pathways (Table 1 ) [ 190 , 204 , 205 ]. In addition, one study found that the antidepressant effects of ketamine were blocked when naltrexone, an opioid antagonist, was administered prior to ketamine [ 206 ], suggesting that the antidepressant effect of ketamine may be dependent on opioid receptor activation and not necessarily due to neurological actions mediated by NMDA receptors. Ketamine’s S-enantiomer, esketamine, was recently approved by the U.S. Food and Drug Administration (FDA) for treatment-resistant depression and MDD with acute suicidal ideation or behavior [ 207 ]. In contrast to standard monoaminergic ADTs, ketamine has demonstrated rapid antidepressant effects that peak at approximately 24 h and are sustained for approximately 1 week after administration in adults with MDD or bipolar depression [ 208 ]. However, the long-term use of ketamine may induce urologic toxicity [ 209 ], and chronic abuse of ketamine can negatively affect brain structure and functioning and cause cognitive impairment [ 210 ].
Newer ADTs targeting dysregulated GABA neurotransmission are also being developed. These include GABA A and GABA B receptor modulators (allosteric modulators, NASs, agonists, and antagonists), and GABAergic interneuron-targeting neuropeptides [ 45 ].
Neuroactive steroids for treatment of depression
Among the more recent additions to the treatment landscape, brexanolone, a NAS GABA A receptor PAM that is chemically identical to endogenous allopregnanolone (Table 1 ), was approved in 2019 by the FDA to treat adults with PPD (Table 2 ) [ 211 ]. This indication was expanded in 2022 to include patients ≥15 years of age [ 212 ]. Prior to the approval of brexanolone, the standard of care for PPD was psychotherapy, psychotropics, or combination treatment. Medications adapted from MDD treatment but not specifically approved for PPD included SSRIs, SNRIs, and TCAs [ 213 ]. In pivotal phase 2 and 3 clinical trials, adult women with PPD who received brexanolone demonstrated significant improvement in depressive symptoms compared with those who received placebo; improvement was rapid (at Hour 60) and sustained (through day 30) [ 214 , 215 , 216 ]. Brexanolone was generally well tolerated in these trials [ 214 , 216 ]. An intravenous preparation of brexanolone was used because of low oral bioavailability and high in vivo clearance of endogenous allopregnanolone [ 216 ]. While the use of brexanolone can be limited by the relatively long, continuous infusion time (60 h), these data have led to an increased interest in the therapeutic potential of GABA A receptor-modulating NASs.
Another NAS, PRAX-114 is a primarily extrasynaptic GABA A receptor PAM in oral formulation that was being investigated for the treatment of MDD [ 217 , 218 ] (Table 2 ). Interim results from a non–placebo-controlled, 3-arm, fixed-dose, phase 2 safety and tolerability study conducted in Australia showed improvements from baseline in depression severity as assessed by HAMD-17 total score reductions following a 14-day treatment course with PRAX-114 [ 219 ]. Changes from baseline (CFB) in the HAMD-17 total score (reductions of 15–19 points) were observed in all 3 arms over an 8-day period. The safety and efficacy of a 28-day treatment course with PRAX-114 as monotherapy for severe MDD were also assessed in the phase 2/3, randomized, double-blind, placebo-controlled Aria trial ( N = 216) [ 217 ]. However, this study failed to meet its primary endpoint of CFB in HAMD-17 total score on day 15, nor did it meet any of the secondary endpoints [ 220 ]. The sponsor has closed screening in its randomized, double-blind, placebo-controlled phase 2 trial as adjunctive and monotherapy treatment for patients with MDD and inadequate response to antidepressant treatment ( N = 110), has stopped enrollment in a PTSD phase 2 trial, and has discontinued an essential tremor trial. The sponsor has no plans to pursue further development of PRAX-114 for psychiatric disorders.
PH10 is an investigational, synthetic NAS from the family of pherines, formulated as a nasal spray, currently under clinical development for treatment of MDD [ 221 ] (Table 2 ). PH10 acts on nasal chemosensory receptors to modulate neural circuits in the brain, including connections to the limbic amygdala and other basal forebrain structures, leading to antidepressant effects [ 221 ]. In a 3-arm (high-dose, low-dose, and placebo) phase 2a pilot study in patients with MDD ( N = 30), treatment with PH10 led to a greater improvement in depressive symptoms as assessed by CFB (reductions) in HAMD-17 total score compared with placebo after 8 weeks of treatment, with minimal side effects and potentially a rapid (week 1) onset of effects [ 221 , 222 ]. Mean CFB in HAMD-17 total score at week 8 were 17.8 (high dose), 16.3 (low dose), and 10.9 for placebo (overall p = 0.07; high dose p = 0.02; low dose p = 0.10). HAMD-17 responder rates of the 3 doses at week 8 were 80% (high dose; p > 0.05), 90% (low dose; p > 0.05), and 60% (placebo), and remission rates were 60% ( p > 0.05), 80% ( p < 0.05), and 20%, respectively. Adverse events that were more common with PH10 compared with placebo included increased appetite, daytime sleepiness, nasal dryness, headache, and bitter taste. A phase 2b trial of PH10 nasal spray for the treatment of MDD has been planned [ 223 ]. In addition, future development as a treatment for PPD, treatment-resistant depression, and suicidal ideation is under consideration [ 224 ].
Zuranolone is an oral, investigational, synthetic NAS and PAM of both synaptic and extrasynaptic GABA A receptors that upregulates GABA A receptor expression and enhances inhibitory GABAergic signaling [ 225 ]. It is currently in clinical development and being investigated as an oral, 14-day treatment for adults with MDD or PPD (Table 2 ). Zuranolone has a pharmacokinetic profile that enables oral once-daily dosing with increased bioavailability [ 226 , 227 ]. In two phase 3 trials in adults with PPD assessing zuranolone 30 mg ( N = 150) or zuranolone 50 mg ( N = 195), those who received a once-daily, 14-day treatment course of zuranolone demonstrated significant improvements in depressive symptoms as assessed by CFB (reductions) in HAMD-17 total score at day 15 compared with those who received placebo [ 228 , 229 ]. Mean CFB in HAMD-17 total score at day 15 were 17.8 (vs placebo 13.6; p < 0.05) with zuranolone 30 mg and 15.6 (vs placebo −11.6; p < 0.05) with zuranolone 50 mg. Rapid (day 3) and sustained (day 45) improvements in depressive symptoms were significantly greater with zuranolone than with placebo ( p < 0.05) in both studies. HAMD-17 response rates at day 45 (end of study) were 75.3% (vs placebo 56.5%; nominal p > 0.05) and 61.9% (vs placebo 54.1%; nominal p > 0.05), and remission rates were 53.4% (vs placebo 30.4%; nominal p < 0.01) and 44.0% (vs placebo 29.4%; nominal p > 0.05) in the 2 studies [ 228 , 229 ]. In a phase 2 trial (zuranolone 30 mg, N = 89) and a phase 3 trial (zuranolone 50 mg, N = 534) in adults with MDD, those who received treatment with zuranolone demonstrated significantly greater improvements in depressive symptoms as assessed by CFB (reductions) in HAMD-17 total score at day 15 compared with those who received placebo [ 230 , 231 ]; mean CFB in HAMD-17 total score at day 15 were 17.4 (vs placebo 10.3; p < 0.05) and 14.1 (vs placebo −12.3; p < 0.05), respectively. Another phase 3 trial assessing zuranolone 20 mg ( N = 194) and 30 mg ( N = 194) in patients with MDD did not meet its primary endpoint [ 232 ]; mean CFB in HAMD-17 total score at day 15 was 12.5 with zuranolone 30 mg compared with 11.1 with placebo ( N = 193) ( p > 0.05). Rapid (by day 2 or 3) improvement in depressive symptoms were observed in these 3 trials in MDD (nominal p < 0.05 vs placebo) [ 233 ]. HAMD-17 response rates at day 42 (end of study) were 61.9% (vs placebo 56.4%; nominal p > 0.05) in the phase 2 trial, 52.9% (vs placebo 45.9%; nominal p > 0.05) in the phase 3 zuranolone 50 mg trial, and 43.4% (vs placebo 41.5%; nominal p > 0.05) in the phase 3 zuranolone 20 or 30 mg trial; HAMD-17 remission rates were 45.2% (vs placebo 33.3%; nominal p > 0.05), 30.8% (vs placebo 29.6%; nominal p > 0.05), and 24.3% (vs placebo 25.9%; nominal p > 0.05), respectively [ 230 , 231 , 232 ]. Use of standard ADTs at baseline was allowed in these trials, providing the patient was on a stable dose. Zuranolone as a co-initiation therapy was evaluated in a phase 3 trial; rapid and significantly greater improvement from baseline in HAMD-17 total score was observed at day 3 with zuranolone versus placebo when co-initiated with standard-of-care ADTs in adults with MDD ( p < 0.001) [ 234 ]. Moreover, in an ongoing open-label study that includes assessment of the need for repeat treatment courses with zuranolone over 1 year, of enrolled adults with MDD who responded at day 15 to treatment (≥50% reduction from baseline in HAMD-17 score) with an initial 14-day treatment course of zuranolone 50 mg and continued beyond day 28, 79.5% received a total of 1 or 2 treatment courses during their time of up to 1 year in the study [ 235 , 236 ]. Zuranolone was generally well tolerated. In clinical trials, adverse events that were more common (>5% in zuranolone) with zuranolone compared with placebo included somnolence, dizziness, sedation, and fatigue. The overall incidence of serious adverse events was low, reported in <2% of zuranolone-treated patients across the trials. No patient enrolled in any clinical trial to date (February 2023) has reported developing withdrawal syndrome after discontinuation of zuranolone.
Conclusions
Treatment responses to standard-of-care oral antidepressants have been suboptimal in many individuals with MDD, potentially due to slow onset of effects, low response rates, adverse effects, and the need for chronic treatment. There remains an unmet need for novel and effective treatments with rapid, robust, and sustained antidepressant effects; with better safety and tolerability than standard-of-care ADTs, and ideally without the need for chronic treatment. The development of novel therapeutics for MDD relies on a deep, comprehensive, and evolving understanding of the pathophysiology of depression.
There has been increased interest in GABA A receptor-based treatment approaches for MDD. Recent research on the proposed mechanism of action of NASs for PPD and MDD underscores the potential role of GABAergic signaling in the pathophysiology of depression. Although the placebo effect in depression may be a factor associated with failure to establish efficacy of novel treatments in clinical trials [ 237 ], data reviewed here indicate that NAS GABA A receptor PAMs may potentially offer rapid and sustained antidepressant benefits for individuals with MDD. Further research is necessary to better understand the role of NAS GABA A receptor PAMs in MDD.
Depression. World Health Organization. Available at https://www.who.int/news-room/fact-sheets/detail/depression . Accessed: March 18.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th edition, text revision (DSM-5-TR) (American Psychiatric Association Publishing, Washington, DC, 2022).
GBD Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–50.
Article Google Scholar
National Survey on Drug Use and Health 2021. Available at https://www.samhsa.gov/data/sites/default/files/reports/rpt39441/NSDUHDetailedTabs2021/NSDUHDetailedTabs2021/NSDUHDetTabsSect6pe2021.htm . Accessed: February 22.
Levey DF, Stein MB, Wendt FR, Pathak GA, Zhou H, Aslan M, et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat Neurosci. 2021;24:954–63.
Article CAS PubMed PubMed Central Google Scholar
Lax E. DNA methylation as a therapeutic and diagnostic target in major depressive disorder. Front Behav Neurosci. 2022;16:759052.
Cheng Z, Su J, Zhang K, Jiang H, Li B. Epigenetic mechanism of early life stress-induced depression: focus on the neurotransmitter systems. Front Cell Dev Biol. 2022;10:929732.
Article PubMed PubMed Central Google Scholar
Reszka E, Jablonska E, Lesicka M, Wieczorek E, Kapelski P, Szczepankiewicz A, et al. An altered global DNA methylation status in women with depression. J Psychiatr Res. 2021;137:283–9.
Article PubMed Google Scholar
Evans JW, Szczepanik J, Brutsche N, Park LT, Nugent AC, Zarate CA Jr. Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol Psychiatry. 2018;84:582–90.
MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–64.
Article CAS PubMed Google Scholar
Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771.
Sarawagi A, Soni ND, Patel AB. Glutamate and GABA homeostasis and neurometabolism in major depressive disorder. Front Psychiatry. 2021;12:637863.
Fischer AS, Keller CJ, Etkin A. The clinical applicability of functional connectivity in depression: pathways toward more targeted intervention. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:262–70.
PubMed Google Scholar
Dai L, Zhou H, Xu X, Zuo Z. Brain structural and functional changes in patients with major depressive disorder: a literature review. PeerJ. 2019;7:e8170.
Jesulola E, Micalos P, Baguley IJ. Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model - are we there yet? Behav Brain Res. 2018;341:79–90.
Li Z, Ruan M, Chen J, Fang Y. Major depressive disorder: advances in neuroscience research and translational applications. Neurosci Bull. 2021;37:863–80.
Javaheripour N, Li M, Chand T, Krug A, Kircher T, Dannlowski U, et al. Altered resting-state functional connectome in major depressive disorder: a mega-analysis from the PsyMRI consortium. Transl Psychiatry. 2021;11:511.
Martino M, Magioncalda P, Huang Z, Conio B, Piaggio N, Duncan NW, et al. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc Natl Acad Sci USA. 2016;113:4824–9.
Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, Shaffer SA, Villamarin V, Tan Y, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44:546–54.
Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562–73.
McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P, et al. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry. 2010;15:237–49.
Luscher B, Fuchs T. GABAergic control of depression-related brain states. Adv Pharm. 2015;73:97–144.
Article CAS Google Scholar
Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharm. 1990;185:1–10.
Adell A. Brain NMDA receptors in schizophrenia and depression. Biomolecules. 2020;10:947.
Wang S, Bian L, Yin Y, Guo J. Targeting NMDA receptors in emotional disorders: their role in neuroprotection. Brain Sci. 2022;12:1329.
Wang J, Wang F, Mai D, Qu S. Molecular mechanisms of glutamate toxicity in Parkinson’s disease. Front Neurosci. 2020;14:585584.
Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–6.
Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–7.
Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. 2019;24:952–64.
Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, et al. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36:198–205.
Lüscher B, Möhler H. Brexanolone, a neurosteroid antidepressant, vindicates the GABAergic deficit hypothesis of depression and may foster resilience. F1000Res. 2019;8:F1000 Faculty Rev-751. Published 2019 May 29.
Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406.
Anderson KM, Collins MA, Kong R, Fang K, Li J, He T, et al. Convergent molecular, cellular, and cortical neuroimaging signatures of major depressive disorder. Proc Natl Acad Sci USA. 2020;117:25138–49.
Li J, Seidlitz J, Suckling J, Fan F, Ji GJ, Meng Y, et al. Cortical structural differences in major depressive disorder correlate with cell type-specific transcriptional signatures. Nat Commun. 2021;12:1647.
Gunduz-Bruce H, Takahashi K, Huang MY. Development of neuroactive steroids for the treatment of postpartum depression. J Neuroendocrinol. 2022;34:e13019.
Maguire J. Neuroactive steroids and GABAergic involvement in the neuroendocrine dysfunction associated with major depressive disorder and postpartum depression. Front Cell Neurosci. 2019;13:83.
Fee C, Banasr M, Sibille E. Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biol Psychiatry. 2017;82:549–59.
Romeo B, Choucha W, Fossati P, Rotge JY. Meta-analysis of central and peripheral gamma-aminobutyric acid levels in patients with unipolar and bipolar depression. J Psychiatry Neurosci. 2018;43:58–66.
Xu JC, Fan J, Wang X, Eacker SM, Kam TI, Chen L, et al. Cultured networks of excitatory projection neurons and inhibitory interneurons for studying human cortical neurotoxicity. Sci Transl Med. 2016;8:333ra348.
Nadadhur AG, Emperador Melero J, Meijer M, Schut D, Jacobs G, Li KW, et al. Multi-level characterization of balanced inhibitory-excitatory cortical neuron network derived from human pluripotent stem cells. PLoS One. 2017;12:e0178533.
Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–53.
Tiwari V, Ambadipudi S, Patel AB. Glutamatergic and GABAergic TCA cycle and neurotransmitter cycling fluxes in different regions of mouse brain. J Cereb Blood Flow Metab. 2013;33:1523–31.
Chang CL, Trimbuch T, Chao HT, Jordan JC, Herman MA, Rosenmund C. Investigation of synapse formation and function in a glutamatergic-GABAergic two-neuron microcircuit. J Neurosci. 2014;34:855–68.
Wu C, Sun D. GABA receptors in brain development, function, and injury. Metab Brain Dis. 2015;30:367–79.
Fogaca MV, Duman RS. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci. 2019;13:87.
Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9:182–94.
Eban-Rothschild A, Appelbaum L, de Lecea L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. 2018;43:937–52.
Cellot G, Cherubini E. Functional role of ambient GABA in refining neuronal circuits early in postnatal development. Front Neural Circuits. 2013;7:136.
Chen X, Fan X, Hu Y, Zuo C, Whitfield-Gabrieli S, Holt D, et al. Regional GABA concentrations modulate inter-network resting-state functional connectivity. Cereb Cortex. 2019;29:1607–18.
Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron. 2016;91:260–92.
Mohler H. Molecular regulation of cognitive functions and developmental plasticity: impact of GABA A receptors. J Neurochem. 2007;102:1–12.
Jewett BE, Sharma S. Physiology, GABA. (StatPearls Publishing, 2021).
Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–8.
Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA B receptors. Physiol Rev. 2004;84:835–67.
Pehrson AL, Sanchez C. Altered gamma-aminobutyric acid neurotransmission in major depressive disorder: a critical review of the supporting evidence and the influence of serotonergic antidepressants. Drug Des Devel Ther. 2015;9:603–24.
Li N, Tao W, Yang L, Spain WJ, Ransom CB. GABA-B receptors enhance GABA-A receptor currents by modulation of membrane trafficking in dentate gyrus granule cells. Neurosci Lett. 2022;773:136481.
Tao W, Higgs MH, Spain WJ, Ransom CB. Postsynaptic GABA B receptors enhance extrasynaptic GABA A receptor function in dentate gyrus granule cells. J Neurosci. 2013;33:3738–43.
Ghit A, Assal D, Al-Shami AS, Hussein DEE. GABA A receptors: structure, function, pharmacology, and related disorders. J Genet Eng Biotechnol. 2021;19:123.
Young AB, Chu D. Distribution of GABA A and GABA B receptors in mammalian brain: potential targets for drug development. Drug Dev Res. 1990;21:161–7.
Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABA(A) receptors: discrete binding sites underlie subtype specificity. Nat Neurosci. 2003;6:362–9.
Mori M, Gähwiler BH, Gerber U. Beta-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro. J Physiol. 2002;539:191–200.
Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA A receptors. Pharm Ther. 2007;116:7–19.
Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, et al. Structure and subunit composition of GABA(A) receptors. Neurochem Int. 1999;34:379–85.
Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–37.
Naffaa MM, Hung S, Chebib M, Johnston GAR, Hanrahan JR. GABA-rho receptors: distinctive functions and molecular pharmacology. Br J Pharm. 2017;174:1881–94.
Morris KD, Moorefield CN, Amin J. Differential modulation of the gamma-aminobutyric acid type C receptor by neuroactive steroids. Mol Pharm. 1999;56:752–9.
CAS Google Scholar
Li W, Jin X, Covey DF, Steinbach JH. Neuroactive steroids and human recombinant rho1 GABAC receptors. J Pharm Exp Ther. 2007;323:236–47.
Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABA A receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–63.
Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle–linked changes in GABA A receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804.
Engin E, Benham RS, Rudolph U. An emerging circuit pharmacology of GABA A receptors. Trends Pharm Sci. 2018;39:710–32.
Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–8.
Tuem KB, Atey TM. Neuroactive steroids: receptor interactions and responses. Front Neurol. 2017;8:442.
Lloyd-Evans E, Waller-Evans H. Biosynthesis and signalling functions of central and peripheral nervous system neurosteroids in health and disease. Essays Biochem. 2020;64:591–606.
Tsutsui K, Ukena K, Usui M, Sakamoto H, Takase M. Novel brain function: biosynthesis and actions of neurosteroids in neurons. Neurosci Res. 2000;36:261–73.
Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–87.
Joels M. Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol. 1997;18:2–48.
Joksimovic SL, Covey DF, Jevtovic-Todorovic V, Todorovic SM. Neurosteroids in pain management: a new perspective on an old player. Front Pharm. 2018;9:1127.
Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABA A receptor subtypes. Nat Rev Drug Discov. 2011;10:685–97.
Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABA A receptors through two discrete transmembrane sites. Nature. 2006;444:486–9.
Zorumski CF, Paul SM, Covey DF, Mennerick S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol Stress. 2019;11:100196.
Dubrovsky BO. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:169–92.
Borowicz KK, Piskorska B, Banach M, Czuczwar SJ. Neuroprotective actions of neurosteroids. Front Endocrinol (Lausanne). 2011;2:50.
Schverer M, Lanfumey L, Baulieu EE, Froger N, Villey I. Neurosteroids: non-genomic pathways in neuroplasticity and involvement in neurological diseases. Pharm Ther. 2018;191:190–206.
Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA A receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–62.
Ratner MH, Kumaresan V, Farb DH. Neurosteroid actions in memory and neurologic/neuropsychiatric disorders. Front Endocrinol (Lausanne). 2019;10:169.
Puia G, Santi M, Vicini S, Pritchett DB, Purdy RH, Paul SM, et al. Neurosteroids act on recombinant human GABA A receptors. Neuron. 1990;4:759–65.
Alvarez LD, Pecci A, Estrin DA. In search of GABAA receptor’s neurosteroid binding sites. J Med Chem. 2019;62:5250–60.
Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, et al. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABA A receptors. Proc Natl Acad Sci USA. 2014;111:7132–7.
Modgil A, Parakala ML, Ackley MA, Doherty JJ, Moss SJ, Davies PA. Endogenous and synthetic neuroactive steroids evoke sustained increases in the efficacy of GABAergic inhibition via a protein kinase C-dependent mechanism. Neuropharmacology. 2017;113:314–22.
Parakala ML, Zhang Y, Modgil A, Chadchankar J, Vien TN, Ackley MA, et al. Metabotropic, but not allosteric, effects of neurosteroids on GABAergic inhibition depend on the phosphorylation of GABA A receptors. J Biol Chem. 2019;294:12220–30.
Reddy DS, Estes WA. Clinical potential of neurosteroids for CNS disorders. Trends Pharm Sci. 2016;37:543–61.
Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA A receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–43.
Antonoudiou P, Colmers PLW, Walton NL, Weiss GL, Smith AC, Nguyen DP, et al. Allopregnanolone mediates affective switching through modulation of oscillatory states in the basolateral amygdala. Biol Psychiatry. 2022;91:283–93.
Northoff G, Sibille E. Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol Psychiatry. 2014;19:966–77.
Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, et al. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry. 2017;81:886–97.
Zhong H, Rong J, Zhu C, Liang M, Li Y, Zhou R. Epigenetic modifications of GABAergic interneurons contribute to deficits in adult hippocampus neurogenesis and depression-like behavior in prenatally stressed mice. Int J Neuropsychopharmacol. 2020;23:274–85.
Poulter MO, Du L, Weaver ICG, Palkovits M, Faludi G, Merali Z, et al. GABA A receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biol Psychiatry. 2008;64:645–52.
Nardone S, Sams DS, Zito A, Reuveni E, Elliott E. Dysregulation of cortical meuron DNA methylation profile in autism spectrum disorder. Cereb Cortex. 2017;27:5739–54.
Costa E, Grayson DR, Guidotti A. Epigenetic downregulation of GABAergic function in schizophrenia: potential for pharmacological intervention? Mol Inter. 2003;3:220–9.
Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 2007;12:385–97.
Guidotti A, Dong E, Gavin DP, Veldic M, Zhao W, Bhaumik DK, et al. DNA methylation/demethylation network expression in psychotic patients with a history of alcohol abuse. Alcohol Clin Exp Res. 2013;37:417–24.
Ho AM, Winham SJ, Armasu SM, Blacker CJ, Millischer V, Lavebratt C, et al. Genome-wide DNA methylomic differences between dorsolateral prefrontal and temporal pole cortices of bipolar disorder. J Psychiatr Res. 2019;117:45–54.
Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, et al. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009;14:175–89.
Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4:e6585.
Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry. 2010;68:512–20.
Smith KS, Rudolph U. Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABA A receptor subtypes. Neuropharmacology. 2012;62:54–62.
Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat Neurosci. 2009;12:438–43.
Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, et al. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol Psychiatry. 2016;80:457–68.
Vollenweider I, Smith KS, Keist R, Rudolph U. Antidepressant-like properties of alpha2-containing GABA A receptors. Behav Brain Res. 2011;217:77–80.
Koester C, Rudolph U, Haenggi T, Papilloud A, Fritschy JM, Crestani F. Dissecting the role of diazepam-sensitive gamma-aminobutyric acid type A receptors in defensive behavioral reactivity to mild threat. Pharm Biochem Behav. 2013;103:541–9.
Maguire J, Mody I. GABA A R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–13.
Melon L, Hammond R, Lewis M, Maguire J. A novel, synthetic, neuroactive steroid Is effective at decreasing depression-like behaviors and improving maternal care in preclinical models of postpartum depression. Front Endocrinol (Lausanne). 2018;9:703.
Acosta GB, Rubio MC. GABA A receptors mediate the changes produced by stress on GABA function and locomotor activity. Neurosci Lett. 1994;176:29–31.
Otero Losada ME. Changes in central GABAergic function following acute and repeated stress. Br J Pharm. 1988;93:483–90.
Czeh B, Vardya I, Varga Z, Febbraro F, Csabai D, Martis LS, et al. Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front Cell Neurosci. 2018;12:148.
Shalaby A, Kamal S. Effect of Escitalopram on GABA level and anti-oxidant markers in prefrontal cortex and nucleus accumbens of chronic mild stress-exposed albino rats. Int J Physiol Pathophysiol Pharm. 2009;1:154–61.
Sherman AD, Petty F. Neurochemical basis of the action of antidepressants on learned helplessness. Behav Neural Biol. 1980;30:119–34.
Petty F, Sherman AD. GABAergic modulation of learned helplessness. Pharm Biochem Behav. 1981;15:567–70.
Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–13.
Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, et al. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–12.
DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19.
Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–82.
Klumpers UM, Veltman DJ, Drent ML, Boellaard R, Comans EF, Meynen G, et al. Reduced parahippocampal and lateral temporal GABA A -[11C]flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging. 2010;37:565–74.
Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry. 2010;67:458–64.
Schur RR, Draisma LW, Wijnen JP, Boks MP, Koevoets MG, Joels M, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of 1 H-MRS studies. Hum Brain Mapp. 2016;37:3337–52.
Godfrey KEM, Gardner AC, Kwon S, Chea W, Muthukumaraswamy SD. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: a systematic review and meta-analysis. J Psychiatr Res. 2018;105:33–44.
Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800.
Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2012;69:139–49.
Gabbay V, Bradley KA, Mao X, Ostrover R, Kang G, Shungu DC. Anterior cingulate cortex gamma-aminobutyric acid deficits in youth with depression. Transl Psychiatry. 2017;7:e1216.
Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol. 2010;13:411–20.
Goddard AW. Cortical and subcortical gamma amino acid butyric acid deficits in anxiety and stress disorders: clinical implications. World J Psychiatry. 2016;6:43–53.
Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77.
Kim YK, Na KS. Role of glutamate receptors and glial cells in the pathophysiology of treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:117–26.
Todd KG, Baker GB. Neurochemical effects of the monoamine oxidase inhibitor phenelzine on brain GABA and alanine: a comparison with vigabatrin. J Pharm Pharm Sci. 2008;11:14s–21s.
Kucukibrahimoglu E, Saygin MZ, Caliskan M, Kaplan OK, Unsal C, Goren MZ. The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur J Clin Pharm. 2009;65:571–7.
Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–5.
Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–9.
Sajdyk T, Johnson P, Fitz S, Shekhar A. Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. J Psychopharmacol. 2008;22:633–41.
Heldt SA, Mou L, Ressler KJ. In vivo knockdown of GAD67 in the amygdala disrupts fear extinction and the anxiolytic-like effect of diazepam in mice. Transl Psychiatry. 2012;2:e181.
Siegel JM. The neurotransmitters of sleep. J Clin Psychiatry. 2004;65:4–7.
CAS PubMed PubMed Central Google Scholar
Winkelman JW, Buxton OM, Jensen JE, Benson KL, O’Connor SP, Wang W, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep. 2008;31:1499–506.
Morgan PT, Pace-Schott EF, Mason GF, Forselius E, Fasula M, Valentine GW, et al. Cortical GABA levels in primary insomnia. Sleep. 2012;35:807–14.
Wang L, Pan Y, Ye C, Guo L, Luo S, Dai S, et al. A network meta-analysis of the long- and short-term efficacy of sleep medicines in adults and older adults. Neurosci Biobehav Rev. 2021;131:489–96.
Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155–62.
McKlveen JM, Moloney RD, Scheimann JR, Myers B, Herman JP. “Braking” the prefrontal cortex: the role of glucocorticoids and interneurons in stress adaptation and pathology. Biol Psychiatry. 2019;86:669–81.
Kamigaki T. Dissecting executive control circuits with neuron types. Neurosci Res. 2019;141:13–22.
Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17:87.
Luchetti S, Huitinga I, Swaab DF. Neurosteroid and GABA-A receptor alterations in Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. Neuroscience. 2011;191:6–21.
Naylor JC, Kilts JD, Szabo ST, Dunn CE, Keefe FJ, Tupler LA, et al. Allopregnanolone levels are inversely associated with self-reported pain symptoms in U.S. Iraq and Afghanistan-era veterans: implications for biomarkers and therapeutics. Pain Med. 2016;17:25–32.
Rasmusson AM, King MW, Valovski I, Gregor K, Scioli-Salter E, Pineles SL, et al. Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology. 2019;102:95–104.
Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–44.
Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, et al. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–3.
Agis-Balboa RC, Guidotti A, Pinna G. 5alpha-reductase type I expression is downregulated in the prefrontal cortex/Brodmann’s area 9 (BA9) of depressed patients. Psychopharmacol (Berl). 2014;231:3569–80.
Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum “blues”. Obstet Gynecol. 2001;97:77–80.
CAS PubMed Google Scholar
Strohle A, Romeo E, di Michele F, Pasini A, Hermann B, Gajewsky G, et al. Induced panic attacks shift gamma-aminobutyric acid type A receptor modulatory neuroactive steroid composition in patients with panic disorder: preliminary results. Arch Gen Psychiatry. 2003;60:161–8.
Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, et al. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–9.
Grobin AC, Morrow AL. 3Alpha-hydroxy-5alpha-pregnan-20-one levels and GABA(A) receptor-mediated 36Cl(−) flux across development in rat cerebral cortex. Brain Res Dev Brain Res. 2001;131:31–39.
Purdy RH, Morrow AL, Moore PH Jr., Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–7.
Maguire J. Stress-induced plasticity of GABAergic inhibition. Front Cell Neurosci. 2014;8:157.
Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, et al. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–54.
Matsumoto K, Pinna G, Puia G, Guidotti A, Costa E. Social isolation stress-induced aggression in mice: a model to study the pharmacology of neurosteroidogenesis. Stress. 2005;8:85–93.
Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci USA. 2008;105:5567–72.
Evans J, Sun Y, McGregor A, Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology. 2012;63:1315–26.
Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, et al. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA A receptor function in rat brain. J Neurochem. 2000;75:732–40.
Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacol (Berl). 2006;186:362–72.
Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci USA. 2003;100:2035–40.
Locci A, Pinna G. Neurosteroid biosynthesis down-regulation and changes in GABA A receptor subunit composition: a biomarker axis in stress-induced cognitive and emotional impairment. Br J Pharm. 2017;174:3226–41.
Vega Alanis BA, Iorio MT, Silva LL, Bampali K, Ernst M, Schnurch M, et al. Allosteric GABA A receptor modulators-a review on the most recent heterocyclic chemotypes and their synthetic accessibility. Molecules. 2020;25:999.
Twyman RE, Rogers CJ, Macdonald RL. Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann Neurol. 1989;25:213–20.
Hanson SM, Czajkowski C. Structural mechanisms underlying benzodiazepine modulation of the GABA(A) receptor. J Neurosci. 2008;28:3490–9.
Bianchi MT, Botzolakis EJ, Lagrange AH, Macdonald RL. Benzodiazepine modulation of GABA(A) receptor opening frequency depends on activation context: a patch clamp and simulation study. Epilepsy Res. 2009;85:212–20.
Baer AB & Holstege CP Barbiturates, long-acting. in Encyclopedia of Toxicology (Second Edition) (ed. Wexler, P) 209-10 (Elsevier, New York, 2005).
Löscher W, Rogawski MA. How theories evolved concerning the mechanism of action of barbiturates. Epilepsia. 2012;53:12–25.
Ciraulo DA & Oldham M Chapter Sixteen - Sedative hypnotics. in The Effects of Drug Abuse on the Human Nervous System (eds. Madras, B & Kuhar, M) 499-532 (Academic Press, Boston, 2014).
Balon R, Starcevic V, Silberman E, Cosci F, Dubovsky S, Fava GA, et al. The rise and fall and rise of benzodiazepines: a return of the stigmatized and repressed. Braz J Psychiatry. 2020;42:243–4.
Petty F, Trivedi MH, Fulton M, Rush AJ. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38:578–91.
Lim B, Sproule BA, Zahra Z, Sunderji N, Kennedy SH, Rizvi SJ. Understanding the effects of chronic benzodiazepine use in depression: a focus on neuropharmacology. Int Clin Psychopharmacol. 2020;35:243–53.
Lader M. Benzodiazepines revisited–will we ever learn? Addiction. 2011;106:2086–109.
Cheng T, Wallace DM, Ponteri B, Tuli M. Valium without dependence? Individual GABA(A) receptor subtype contribution toward benzodiazepine addiction, tolerance, and therapeutic effects. Neuropsychiatr Dis Treat. 2018;14:1351–61.
Dodds TJ. Prescribed benzodiazepines and suicide risk: a review of the literature. Prim Care Companion CNS Disord. 2017;19(2). https://doi.org/10.4088/PCC.16r02037 .
McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–92.
Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. Br J Pharm. 2000;131:1251–4.
Fiorelli R, Rudolph U, Straub CJ, Feldon J, Yee BK. Affective and cognitive effects of global deletion of alpha3-containing gamma-aminobutyric acid-A receptors. Behav Pharm. 2008;19:582–96.
Engin E, Liu J, Rudolph U. α2-containing GABA(A) receptors: a target for the development of novel treatment strategies for CNS disorders. Pharm Ther. 2012;136:142–52.
Martinez Botella G, Salituro FG, Harrison BL, Beresis RT, Bai Z, Blanco MJ, et al. Neuroactive steroids. 2. 3alpha-hydroxy-3beta-methyl-21-(4-cyano-1H-pyrazol-1’-yl)-19-nor-5beta-pregnan-20 -one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (gamma-Aminobutyric Acid)A receptor. J Med Chem. 2017;60:7810–9.
Jacob TC, Michels G, Silayeva L, Haydon J, Succol F, Moss SJ. Benzodiazepine treatment induces subtype-specific changes in GABA A receptor trafficking and decreases synaptic inhibition. Proc Natl Acad Sci USA. 2012;109:18595–600.
Wlodarczyk AI, Sylantyev S, Herd MB, Kersante F, Lambert JJ, Rusakov DA, et al. GABA-independent GABA A receptor openings maintain tonic currents. J Neurosci. 2013;33:3905–14.
Santarsieri D, Schwartz TL. Antidepressant efficacy and side-effect burden: a quick guide for clinicians. Drugs Context. 2015;4:212290.
Mantas I, Saarinen M, Xu ZD, Svenningsson P. Update on GPCR-based targets for the development of novel antidepressants. Mol Psychiatry. 2022;27:534–58.
Aleksandrova LR, Phillips AG, Wang YT. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J Psychiatry Neurosci. 2017;42:222–9.
Depression Medicines. U. S. Food and Drug Administration Office of Women’s Health. Available at https://www.fda.gov/consumers/free-publications-women/depression-medicines . Accessed: November 13, 2022.
Protti M, Mandrioli R, Marasca C, Cavalli A, Serretti A, Mercolini L. New-generation, non-SSRI antidepressants: Drug-drug interactions and therapeutic drug monitoring. Part 2: NaSSAs, NRIs, SNDRIs, MASSAs, NDRIs, and others. Med Res Rev. 2020;40:1794–832.
Mandrioli R, Protti M, Mercolini L. New-generation, non-SSRI antidepressants: therapeutic drug monitoring and pharmacological interactions. Part 1: SNRIs, SMSs, SARIs. Curr Med Chem. 2018;25:772–92.
Sheffler ZM, Patel P & Abdijadid S. Antidepressants. in StatPearls (Treasure Island (FL), 2022).
Chu A & Wadhwa R. Selective serotonin reuptake inhibitors. in StatPearls (Treasure Island (FL), 2021).
Lambert O, Bourin M. SNRIs: mechanism of action and clinical features. Expert Rev Neurother. 2002;2:849–58.
Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000;58:19–36.
White N, Litovitz T, Clancy C. Suicidal antidepressant overdoses: a comparative analysis by antidepressant type. J Med Toxicol. 2008;4:238–50.
Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21:454–64.
Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pr. 2004;10:239–48.
Google Scholar
De Crescenzo F, Perelli F, Armando M, Vicari S. Selective serotonin reuptake inhibitors (SSRIs) for post-partum depression (PPD): a systematic review of randomized clinical trials. J Affect Disord. 2014;152–154:39–44.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17.
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40.
Matveychuk D, Thomas RK, Swainson J, Khullar A, MacKay MA, Baker GB, et al. Ketamine as an antidepressant: overview of its mechanisms of action and potential predictive biomarkers. Ther Adv Psychopharmacol. 2020;10:2045125320916657.
Royo M, Escolano BA, Madrigal MP, Jurado S. AMPA receptor function in hypothalamic synapses. Front Synaptic Neurosci. 2022;14:833449.
Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205–15.
Janssen Pharmaceuticals Inc. SPRAVATO ® (esketamine) nasal spray, CIII [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals, Inc. (2020).
Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46:1459–72.
Winstock AR, Mitcheson L, Gillatt DA, Cottrell AM. The prevalence and natural history of urinary symptoms among recreational ketamine users. BJU Int. 2012;110:1762–6.
Strous JFM, Weeland CJ, van der Draai FA, Daams JG, Denys D, Lok A, et al. Brain changes associated with long-term ketamine abuse, a systematic review. Front Neuroanat. 2022;16:795231.
SAGE Therapeutics Inc. ZULRESSO (brexanolone) injection for intravenous use [prescribing information]. Cambridge, MA: SAGE Therapeutics, Inc. 2019;
Sage Therapeutics Inc. ZULRESSO (brexanolone) injection for intravenous use [prescribing information]. Cambridge, MA: Sage Therapeutics Inc. 2022;
Frieder A, Fersh M, Hainline R, Deligiannidis KM. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS drugs. 2019;33:265–82.
Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058–70.
Gerbasi ME, Meltzer-Brody S, Acaster S, Fridman M, Bonthapally V, Hodgkins P, et al. Brexanolone in postpartum depression: post hoc analyses to help inform clinical decision-making. J Women’s Health (Larchmt). 2021;30:385–92.
Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390:480–9.
A clinical trial of PRAX-114 in participants with major depressive disorder. ClinicalTrials.gov identifier: NCT04832425. Available at https://clinicaltrials.gov/ct2/show/NCT04832425?term=Prax+114&draw=2&rank=2 . Accessed: June 10.
A clinical trial of adjunctive and monotherapy PRAX-114 in participants with major depressive disorder. ClinicalTrials.gov identifier: NCT04969510. Available at https://clinicaltrials.gov/ct2/show/NCT04969510?term=Prax+114&draw=2&rank=3 . Accessed: June 10.
Hecking J, Davoudian PA, Wilkinson ST. Emerging therapeutics based on the amino acid neurotransmitter system: an update on the pharmaceutical pipeline for mood disorders. Chronic Stress (Thousand Oaks). 2021;5:24705470211020446.
Praxis Precision Medicines reports negative results from PRAX-114 phase 2/3 monotherapy Aria Study in patients with major depressive disorder. News release. Praxis Precision Medicines. June 6, 2022. Accessed March 14, 2023. https://investors.praxismedicines.com/news-releases/news-release-details/praxis-precision-medicines-reports-negative-results-prax-114 .
Monti L, Nicolini H, Liebowitz MR, Hanover R. A placebo controlled trial of PH10: test of a rapidly acting intranasally administered antidepressant. Br J Pharm Med Res. 2019;4:12.
VistaGen announces positive results of newly published exploratory phase 2a study of PH10 for rapid-onset treatment of major depressive disorder. News release. VistaGen Therapeutics. Februrary 20, 2020. Accessed March 14, 2023. https://www.vistagen.com/news-releases/news-release-details/vistagen-announces-positive-results-newly-published-exploratory .
VistaGen’s PH10 nasal spray demonstrates different mechanism of action from benzodiazepines in preclinical study. News release. VistaGen Therapeutics. March 11, 2021. Accessed March 14, 2023. https://www.vistagen.com/news-releases/news-release-details/vistagens-ph10-nasal-spray-demonstrates-different-mechanism
Pipeline overview. Available at https://www.vistagen.com/pipeline/overview . Accessed: June 24, 2022.
Althaus AL, Ackley MA, Belfort GM, Gee SM, Dai J, Nguyen DP, et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABA A receptor positive allosteric modulator. Neuropharmacology. 2020;181:108333.
Hoffmann E, Nomikos GG, Kaul I, Raines S, Wald J, Bullock A, et al. SAGE-217, a novel GABA A receptor positive allosteric modulator: clinical pharmacology and tolerability in randomized phase I dose-finding studies. Clin Pharmacokinet. 2020;59:111–20.
Smith DA, Beaumont K, Maurer TS, Di L. Relevance of half-life in drug design. J Med Chem. 2018;61:4273–82.
Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, Doherty J, Jonas J, Li S, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78:951–9.
Deligiannidis K, Meltzer-Brody S, Maximos B, et al. Zuranolone for the treatment of adults with postpartum depression. Am J Psychiatry. 2023. In press.
Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, et al. Trial of SAGE-217 in patients with major depressive disorder. N. Engl J Med. 2019;381:903–11.
Clayton AH, Lasser R, Parikh SV, Iosifescu DV, Jung J, Kotecha M, et al. Zuranolone for the treatment of adults with major depressive disorder: a phase 3, randomized, placebo‐controlled trial. Am J Psychiatry. 2023;appiajp20220459. https://doi.org/10.1176/appi.ajp.20220459 .
Clayton AH, Lasser R, Nandy I, Sankoh AJ, Jonas J, Kanes SJ. Zuranolone in major depressive disorder: results from MOUNTAIN—a phase 3, multicenter, double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2023;84:22m14445.
Clayton AH, Deligiannidis KM, Kotecha M, Doherty J. Overview of the rapid antidepressant effects observed in the zuranolone clinical development program [A164]. Obstet Gynecol. 2022;139:47S–48S.
Sage Therapeutics and Biogen announce the phase 3 CORAL Study met its primary and key secondary endpoints - comparing zuranolone 50 mg co-initiated with standard of sare antidepressant vs. standard of care co-initiated with placebo in people with MDD. News release. Sage Therapeutics Inc. Februrary 16, 2022. Accessed March 14, 2023. https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-and-biogen-announce-phase-3-coral-study-met .
Sage Therapeutics announces continued positive zuranolone data for both 30 mg and 50 mg doses in open-label SHORELINE Study in patients with MDD. News release. Sage Therapeutics Inc. March 17, 2021. Accessed March 14, 2023. https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-announces-continued-positive-zuranolone-data .
Sage Therapeutics and Biogen announce positive, one-year zuranolone 50 mg data in the ongoing open-label SHORELINE Study in patients with MDD. News release. Biogen Inc. December 1, 2021. Accessed March 14, 2023. https://investors.biogen.com/news-releases/news-release-details/sage-therapeutics-and-biogen-announce-positive-one-year .
Jones BDM, Razza LB, Weissman CR, Karbi J, Vine T, Mulsant LS, et al. Magnitude of the placebo response across treatment modalities used for treatment-resistant depression in adults: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:e2125531.
Dell’Osso B, Palazzo MC, Oldani L, Altamura AC. The noradrenergic action in antidepressant treatments: pharmacological and clinical aspects. CNS Neurosci Ther. 2011;17:723–32.
Moraczewski J & Aedma KK. Tricyclic antidepressants. in StatPearls (Treasure Island (FL), 2021).
Sub Laban T & Saadabadi A. Monoamine oxidase inhibitors (MAOI). in StatPearls (Treasure Island (FL), 2021).
Sabri MA & Saber-Ayad MM. MAO Inhibitors. in StatPearls (Treasure Island (FL), 2022).
Nguyen L, Robson MJ, Healy JR, Scandinaro AL, Matsumoto RR. Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. PLoS One. 2014;9:e89985.
Iosifescu DV, Jones A, O’Gorman C, Streicher C, Feliz S, Fava M, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83:21m14345.
Schwartz J, Murrough JW, Iosifescu DV. Ketamine for treatment-resistant depression: recent developments and clinical applications. Evid Based Ment Health. 2016;19:35–38.
Download references
Acknowledgements
Medical writing and editorial assistance were provided by Symbiotix, LLC (Linda M Ritter, PhD, Medical Director) and funded by Sage Therapeutics, Inc., and Biogen Inc.
Author information
Authors and affiliations.
SUNY Upstate Medical University, Syracuse, NY, USA
Andrew J. Cutler
Washington University, St. Louis, MO, USA
Gregory W. Mattingly
University of South Carolina, Columbia, SC, USA
Vladimir Maletic
You can also search for this author in PubMed Google Scholar
Contributions
AJC, GWM, and VM were involved in the conceptualization, writing, and editing of the paper.
Corresponding author
Correspondence to Andrew J. Cutler .
Ethics declarations
Competing interests.
AJC serves as a consultant to AbbVie, Acadia, AiCure, Alfasigma, Alkermes, Axsome, BioXcel, Boehringer Ingelheim, Cognitive Research, Corium, Intra-Cellular Therapies, Janssen, Jazz Pharmaceuticals, Lundbeck, MedAvante-Prophase, Neurocrine, Noven, Otsuka, Relmada, Sage Therapeutics, Inc., Sunovion, Supernus, Takeda, and Teva. Dr. Cutler serves as a speaker for/receives promotional honoraria from AbbVie, Acadia, Alfasigma, Alkermes, Axsome, Corium, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Sunovion, Supernus, Takeda, and Teva. He has received research grants from Acadia, Alkermes, Allergan, Axsome, Biohaven, Intra-Cellular Therapies, Janssen, Lilly, Lundbeck, Novartis, Otsuka, Sage Therapeutics, Inc., Sunovion, and Takeda. Dr. Cutler is also an employee and board member of the Neuroscience Education Institute. GWM serves as a researcher for AbbVie, Akili, Alkermes, Axsome, Boehringer Ingelheim, Genentech, Janssen, Lundbeck, Medgenics, NLS Pharma, Otsuka, Reckitt Benckiser, Roche, Sage Therapeutics, Inc., Sunovion, Supernus, Takeda, Taisho, and Teva. Dr. Mattingly serves as a consultant for AbbVie, Alkermes, Alfasigma, Ironshore, Janssen, Lundbeck, Major League Baseball, Otsuka, National Football League, Neos, NLS Pharma, Purdue, Rhodes, Sage Therapeutics, Inc., Sunovion, Supernus, Takeda, Teva, and Vanda. Additionally, he serves as a speaker for AbbVie, Alkermes, Ironshore, Janssen, Lundbeck, Otsuka, Neos, Shire, Sunovion, Takeda, and Teva. VM serves as a consultant for AbbVie/Allergan, Acadia Pharmaceuticals, Inc., Alfasigma, USA, Inc., Alkermes, Inc., Eisai, Intra-Cellular Therapies, Ironshore, Janssen, Lundbeck A/S, Jazz Pharmaceuticals, Noven Pharmaceuticals Inc, Otsuka America Pharmaceutical, Inc., Sage Therapeutics, Inc., Sunovion, Supernus, and Takeda. Dr. Maletic also serves as a speaker for AbbVie, Acadia, Alfasigma, Alkermes, Allergan, Eisai, Ironshore, Intra-Cellular, Janssen, H. Lundbeck A/S, Otsuka America Pharmaceutical, Inc., Sunovion, Supernus, and Takeda.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cutler, A.J., Mattingly, G.W. & Maletic, V. Understanding the mechanism of action and clinical effects of neuroactive steroids and GABAergic compounds in major depressive disorder. Transl Psychiatry 13 , 228 (2023). https://doi.org/10.1038/s41398-023-02514-2
Download citation
Received : 16 November 2022
Revised : 12 May 2023
Accepted : 12 June 2023
Published : 26 June 2023
DOI : https://doi.org/10.1038/s41398-023-02514-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Reference Manager
- Simple TEXT file
People also looked at
Review article, cortical gabaergic dysfunction in stress and depression: new insights for therapeutic interventions.

- Department of Psychiatry, Yale University School of Medicine, New Haven, CT, United States
Major depressive disorder (MDD) is a debilitating illness characterized by neuroanatomical and functional alterations in limbic structures, notably the prefrontal cortex (PFC), that can be precipitated by exposure to chronic stress. For decades, the monoaminergic deficit hypothesis of depression provided the conceptual framework to understand the pathophysiology of MDD. However, accumulating evidence suggests that MDD and chronic stress are associated with an imbalance of excitation–inhibition (E:I) within the PFC, generated by a deficit of inhibitory synaptic transmission onto principal glutamatergic neurons. MDD patients and chronically stressed animals show a reduction in GABA and GAD67 levels in the brain, decreased expression of GABAergic interneuron markers, and alterations in GABA A and GABA B receptor levels. Moreover, genetically modified animals with deletion of specific GABA receptors subunits or interneuron function show depressive-like behaviors. Here, we provide further evidence supporting the role of cortical GABAergic interneurons, mainly somatostatin- and parvalbumin-expressing cells, required for the optimal E:I balance in the PFC and discuss how the malfunction of these cells can result in depression-related behaviors. Finally, considering the relatively low efficacy of current available medications, we review new fast-acting pharmacological approaches that target the GABAergic system to treat MDD. We conclude that deficits in cortical inhibitory neurotransmission and interneuron function resulting from chronic stress exposure can compromise the integrity of neurocircuits and result in the development of MDD and other stress-related disorders. Drugs that can establish a new E:I balance in the PFC by targeting the glutamatergic and GABAergic systems show promising as fast-acting antidepressants and represent breakthrough strategies for the treatment of depression.
Introduction
Major depressive disorder (MDD) is a recurring neuropsychiatric illness that is among the leading contributors to social and economic burden, rm resilience to stress-induced affecting approximately one in five people in the United States ( Kessler et al., 2005 ). The World Health Organization estimates that MDD will be the second leading cause of disability by 2020 ( Reddy, 2010 ). Moreover, MDD induces a high level of personal suffering and suicidality, and also increases the risk of other comorbid medical conditions that can lead to further disability or death ( Karch et al., 2009 ).
The current pharmacological treatment approaches set by national and international guidelines recommend the use of monoaminergic-based drugs, notably, serotonin reuptake inhibitors (SSRI), as first-line medications ( Bauer et al., 2007 ). Although these drugs provide a significant therapeutic benefit, they still require weeks to months to induce an antidepressant response, and up to 33% of the patients are considered treatment resistant (i.e., fail to respond to two or more antidepressants). Also, the majority of patients experience recurrence after interrupting the treatment, and the adherence of patients to these medicines is relatively low, as they cause undesired side effects, including weight gain, sexual dysfunction, disruption of normal sleep patterns, memory deficits, and others. Moreover, most patients experience a worsening of symptoms in the first weeks of administration, which also contributes to the low treatment adherence ( Trivedi et al., 2006 ).
Since the late 1980s, when the SSRIs were developed and the monoamine deficiency hypothesis of depression gained more support, there have been no considerable advances in pharmacological treatment for MDD. Considering the relatively low efficacy of monoaminergic drugs, there is an urgent need for development of novel medicaments that address current therapeutic limitations. Indeed, in recent years, a new class of fast-acting, efficacious antidepressants has emerged, showing immense promise in clinical and pre-clinical studies. Among them, ketamine, an NMDA receptor blocker, is the most studied due to its fast (within 2 h of administration) and sustained (up to 7 days) antidepressant effects ( Berman et al., 2000 ; Zarate et al., 2006 ; Harmer et al., 2017 ). These glutamatergic-based drugs have shed light on yet unexplored avenues to explain the pathophysiology of depression, shifting efforts to the discovery and development of new classes of drugs. The accumulating evidence in the literature relating stress, GABA/glutamate deficits in the brain, and MDD, as well as the antidepressant efficacy of drugs that directly interact with these systems, have led to alternative hypotheses to explain the complex neurobiology of affective disorders that overcome inconsistences in monoaminergic theories.
In this review, we focus on the GABAergic deficit/imbalance hypothesis of MDD ( Luscher et al., 2011 ; Fee et al., 2017 ), and elaborate this theory in the context of the glutamatergic hypothesis and the monoaminergic and neurotrophic deficits theories. We discuss the dysregulation of GABA neurotransmission and changes in specific GABAergic interneuron subtypes observed in MDD subjects and stress- or genetic-based animal models of depression. Although depression must be seen as a system-wide disorder, a broad range of GABA interneurons that orchestrate excitation–inhibition (E:I) balance in corticolimbic structures are located in the prefrontal cortex (PFC) and several studies point to this region as one of the primary brain regions involved in the pathophysiology of MDD. Indeed, multiple reports show a direct correlation between chronic stress and depression with decreased volume, synaptic atrophy/loss, and altered connectivity in the PFC ( Duman et al., 2016 ). For this reason, we discuss in more detail evidence for cortical impairments in depression. Finally, based on recent findings, we will discuss how monoaminergic drugs can also modulate the GABAergic system and will explore novel non-monoaminergic fast-acting pharmacological approaches to treat MDD, including GABA A and GABA B receptor modulators (allosteric modulators, neurosteroids, agonists, and antagonists), NMDA receptors blockers (such as ketamine), and GABAergic interneurons-targeting neuropeptides.
GABAergic System in the PFC
GABA is the major inhibitory mediator of cortical interneurons in the brain that serves to modulate a wide range of local neurotransmitter systems, most notably, the glutamatergic excitatory counterpart. By targeting specific somatic domains of neighboring glutamatergic principal neurons, GABA interneurons control the E:I balance in the PFC as well as the excitatory output to projecting areas, such as the amygdala, bed nucleus of stria terminalis, and dorsal raphe nucleus. Due to this network orchestration of firing patterns, cortical GABAergic interneurons play an essential role in mediating complex emotional and cognitive processes in the brain. GABA is synthesized from glutamate by glutamate decarboxylase enzymes (GAD65 and GAD67) and stored in vesicles through the vesicular GABA transporter (VGAT1 and 2). The GABAergic signal is terminated by rapid uptake of GABA to glial cells and presynaptic neurons through plasma membrane GABA transporters (GAT1-4).
One-third of all synapses in the central nervous system (CNS) connects via GABA interneurons, which comprise 20–30% of neocortical neurons and can be classified accordingly to their diverse morphological, electrophysiological, and molecular characteristics ( Markram et al., 2004 ; Rudy et al., 2011 ; DeFelipe et al., 2013 ; Tremblay et al., 2016 ). The most common nomenclature segregates interneurons accordingly to their expression profile of neurochemical markers. Three major non-overlapping interneuron groups in the neocortex include those that express the calcium-binding protein parvalbumin (PV), the neuropeptide somatostatin (SST), and the ionotropic serotonin receptor 3 ( Rudy et al., 2011 ). These neurons can further co-express other markers, such as the neuropeptides cholecystokinin (CCK), vasoactive intestinal peptide (VIP), and neuropeptide Y, as well as other calcium-binding proteins, such as calbindin and calretinin ( Rudy et al., 2011 ). Characterization of distinct subtypes of interneurons helps to identify vulnerable subpopulations that could be relevant to different neuropsychiatric disorders. Specifically, PV and SST interneurons have been extensively studied in stress-related disorders. The most abundant subtype, PV, correspond to 40% of cortical GABA interneurons, and have chandelier or, most commonly, basket cell morphology. PV basket interneurons mainly control firing synchronization and spike timing of neighboring excitatory neurons by providing somatic fast-spiking inhibition to pyramidal cells ( Markram et al., 2004 ; Ferguson and Gao, 2018 ). On the other hand, 25–30% of cortical interneurons express SST, which consist mainly of Marinotti cells with low-threshold regular spiking properties and an independent high basal firing activity. SST cells make synapses on the dendritic tufts of pyramidal cells but can also inhibit local PV interneurons ( Markram et al., 2004 ; Urban-Ciecko and Barth, 2016 ). These distinct properties and sub-localization confer to both SST and PV interneurons different roles in the cortical microcircuit: while SST cells control the spiking inputs to pyramidal neurons, PV interneurons regulate the spiking outputs from pyramidal neurons to projecting brain areas.
GABA interneurons express two subtypes of GABA receptors: GABA A and GABA B . The most prominent receptor, GABA A , is a ligand-gated Cl - ion channel (ionotropic) and has been extensively characterized as the target of many psychotropic agents, including benzodiazepines, ethanol, and barbiturates. These receptors are mostly located post-synaptically and control fast synaptic inhibition. GABA A receptors are tetrameric or pentameric in structure that are made up of multiple subunits (6α, 4β, and 3γ) in distinctive combinations that assemble together around a central chloride pore ( Engin et al., 2018 ). GABA B receptors are Gi-coupled receptors (metabotropic) and composed of a heterodimer of two homologous subunits: GABA B1 and GABA B2 ; they are mainly located at pre-synaptic sites, functioning as autoreceptors and inhibiting GABA release, although they can also be found post-synaptically ( Cryan and Kaupmann, 2005 ). Given the broad spectrum of neuronal activity controlled by GABA interneurons, it is increasingly clear that imbalance in the GABAergic system and hence in the E:I balance can contribute to the pathophysiology of several psychiatric disorders, including MDD.
Cortical Dysregulation of GABA Neurotransmission in Chronic Stress and Depression
Although the adaptive, innate stress response is essential for body homeostasis and survival, it is widely recognized that responses to sustained, chronic stress can become dysregulated and result in illness and abnormal behaviors. In the brain, chronic stress can produce changes in neurotransmitter function and appropriate neuroplasticity responses that could precipitate depression in humans and, therefore, has been extensively used as a rodent model for depression ( Duman et al., 2016 ).
GABAergic neurons play an important role in the termination of stress response through regulation of the hypothalamus–pituitary–adrenal (HPA) axis, and disruption of this regulatory response contributes to the abnormal effects of chronic stress exposure. For example, chronic stress causes down-regulation of the transmembrane K-Cl cotransporter (KCC2), rendering GABA inputs ineffective to synaptic inhibition of the HPA axis ( Hewitt et al., 2009 ). Moreover, deletion or mutation of the γ2 subunit of GABA A receptors (heterozygous γ2 knockout: γ2+/-) result in reduced GABA A receptor binding and consequent HPA axis hyperactivity, leading to anxiogenic and pro-depressive behaviors ( Crestani et al., 1999 ; Chandra et al., 2005 ; Shen et al., 2010 ; Smith and Rudolph, 2012 ). A similar pro-depressive profile is found in α2 knockout mice ( Vollenweider et al., 2011 ). Therefore, genetic modifications in GABA A receptors subunits have been increasingly used as animal models to study the influence of GABAergic system in the pathophysiology of anxiety and depression, as well as pharmacological approaches that have therapeutic potential.
Considering that GABA receptors are highly expressed and GABAergic interneurons are abundant in the PFC and exert an important GABAergic inhibitory control over HPA axis activity ( Diorio et al., 1993 ; Akana et al., 2001 ; Radley et al., 2009 ) it is conceivable that the PFC GABAergic system plays an essential role in emotional processing that is vulnerable to stress. In this respect, acute psychological stress (threat-of-shock condition) decreased approximately 18% of PFC GABA levels relative to a “safe” condition in healthy subjects ( Hasler et al., 2010 ). In rodents, repeated immobilization stress increased GAD activity and GABA turnover, and reduced GABA levels in the frontal cortex ( Otero Losada, 1988 ), an effect that was also reported after a 3-week of chronic mild stress (CMS) exposure ( Shalaby and Kamal, 2009 ). In a learned helplessness paradigm, a model of depression, rats that failed to learn the shuttlebox task showed a 25% reduction of GABA A receptors in cortical synaptoneurosomes ( Drugan et al., 1989 ). Similar results were observed after other types of chronic stress, such as cold and isolation ( Braestrup et al., 1979 ). A recent study reported that 9-weeks CMS exposure resulted in decreased cortical GABA A receptor function, decreased release probability at peri-somatic GABAergic synapses, and reduced postsynaptic GABA B receptor mediated inhibition in anhedonic rats, leading to higher excitability of pyramidal neurons ( Czéh et al., 2018 ). Also, chronic unpredictable stress (CUS) or CMS exposure decreased innervation and function of GABAergic axons, and levels of GAD67, VGAT, and GAT3 in the PFC ( Gilabert-Juan et al., 2013 ; Ma et al., 2016 ; Banasr et al., 2017 ). Besides chronic stress exposure of adult animals, there is also evidence that early life stress exposure impacts the GABAergic system later in life in the adult brain. Maternal separation stress and alteration of maternal care in the early (first weeks) postnatal period decreased expression of GABA A receptors in the frontal cortex and other limbic areas, as well as induced anxiety and depressive-like behaviors in adulthood ( Caldji et al., 2000 , 2003 ).
Collectively, these data provide support for the hypothesis that stress causes major changes in the GABAergic system in the PFC that could result in abnormal behavioral and synaptic responses, including dendritic reorganization of interneurons ( Gilabert-Juan et al., 2013 ), as well as alterations of electrophysiological respones ( Northoff and Sibille, 2014 ; McKlveen et al., 2016 ), that results in defective output from pyramidal neurons to other brain areas. However, even though numerous reports suggest that chronic stress decreases GABA levels and function, other studies have reported opposite effects. Chronic immobilization stress induced a small increase in GABA A receptor binding in the frontal cortex ( Braestrup et al., 1979 ) and chronic social defeat stress increased GABA A -containing α5 subunit in the PFC and hippocampus of susceptible mice ( Xiong et al., 2018 ). Likewise, chronic restraint stress (21 days) induced an increase in GABA A -α1 subunit mRNA expression in the mPFC but not α2, α3, α4, or γ2 ( Gilabert-Juan et al., 2013 ). Moreover, McKlveen et al. (2016) found that CUS (14 days) increased the frequency of miniature inhibitory postsynaptic currents in the infralimbic area, as well as inhibitory appositions and terminals onto glutamatergic cells, suggesting a stress-induced enhancement of prefrontal inhibition. While difficult to reconcile, it is important to highlight that the results of stress studies may differ depending on the type and duration of the stressor, the GABA receptor subunit analyzed, and the specific subregions of the PFC studied.
In addition to these preclinical studies, there is accumulating evidence that dysfunction of the GABAergic system is associated with the pathophysiology of MDD and that normalization of GABA is associated with the remission of depressive symptoms ( Godfrey et al., 2018 ). Pioneering studies showed that patients with depression have lower GABA levels in the plasma ( Petty and Sherman, 1984 ) and the cerebrospinal fluid (CSF) ( Gold et al., 1980 ; Gerner and Hare, 1981 ). Further studies extended this work through positron emission tomography (PET) imaging methods, which permits a direct and noninvasive quantification of GABA levels in the brain. These studies showed that GABA levels are reduced in unmedicated patients with MDD in several cortical areas, including the prefrontal ( Hasler et al., 2007 ), occipital ( Sanacora et al., 1999 , 2004 ; Song et al., 2012 ), and anterior cingulate (ACC) cortices ( Gabbay et al., 2012 ; Godfrey et al., 2018 ). Significant reduction in the ratio GABA/creatine + phosphocreatine was found in the ACC of female veterans with suicidal behavior ( Prescot et al., 2018 ). Likewise, reduced GAD67 protein or gene expression were found in the dorsolateral PFC (dlPFC) and subgenual ACC (SgACC) of depressed patients ( Karolewicz et al., 2010 ; Tripp et al., 2012 ), although other studies reported no significant effects ( Sibille et al., 2011 ; Gilabert-Juan et al., 2012 ). Besides GABA levels, several studies reported decreased expression of GABA A receptors subunit genes in MDD cortices, including decreased α1, α3, α4, γ1, β2, and ρ1 ( Merali et al., 2004 ; Sequeira et al., 2007 ; Klempan et al., 2009 ; Luscher et al., 2011 ). However, there were also reports of increased expression of certain subunits, including α5, γ2, β3, and δ in MDD subjects ( Merali et al., 2004 ; Choudary et al., 2005 ; Sequeira et al., 2007 ; Klempan et al., 2009 ) suggesting that different GABA A receptor subunits may play distinct roles in the etiology of MDD.
Studies regarding the participation of GABA B receptors in the pathophysiology of MDD have received less attention and, therefore, the literature remains unclear. Although GABA B1 and GABA B2 subunits were reported to be decreased in the lateral cerebellum of MDD subjects ( Fatemi et al., 2011 ), no evidence was found for altered GABA B receptor binding in the frontal cortex or hippocampus ( Cross et al., 1988 ; Arranz et al., 1992 ). However, some variables should be considered as potential confounds in this study, as some of the MDD patients were taking antidepressants at the time of death, and in some cases there was a long post-mortem interval before tissue collection. Despite these limitations, it is notable that preclinical studies report that helpless rats showed decreased expression of GABA B receptors in the frontal cortex ( Martin et al., 1989 ), and GABA B1 subunit knockout animals displayed antidepressant-like responses in the forced swim test ( Mombereau et al., 2004 ). Taken together, the results demonstrate that modulation of GABA B receptors induces antidepressant effects (see the section “Conclusion and Future Directions”), and warrant additional studies with more cutting edge tools to further investigate the role of GABA B receptors in depression and treatment response.
GABA Interneuron-Related Deficits in Depression
Numerous studies suggest that the reduction in cortical GABA levels observed in MDD subjects and stressed rodents could not only result from decreased levels of the GABA synthetic enzymes GAD65/67, but could also result from a reduction in the density of specific GABA interneuron subpopulations ( Table 1 ). MDD patients show a reduced volume of brain areas such as the PFC and hippocampus ( MacQueen et al., 2008 ; Savitz and Drevets, 2009 ). Also, abnormalities in the GABAergic system in cortical areas can also robustly affect other brain regions. For example, low GABA levels in the ACC of MDD patients are associated with reduction in hippocampal volume ( Abdallah et al., 2015 ). Reduced SST gene expression, mRNA, or protein levels were found in the CSF, SgACC, dlPFC, and amygdala of MDD subjects, and in the medial PFC (mPFC) and hippocampus of animals exposed to CUS ( Rubinow et al., 1985 ; Rajkowska et al., 2007 ; Sibille et al., 2011 ; Tripp et al., 2011 ; Guilloux et al., 2012 ; Banasr et al., 2017 ). Interestingly, female MDD subjects show a more robust reduction in SST expression than males ( Sibille et al., 2011 ; Tripp et al., 2011 , 2012 ; Guilloux et al., 2012 ), suggesting that SST could be related to the twofold greater incidence of MDD in females ( Kuehner, 2017 ). Although SST expression in MDD subjects and chronically stressed animals is decreased, Gilabert-Juan et al. (2013) reported dendritic hypertrophy of Martinotti cells (which includes SST-expressing interneurons) in the mPFC of mice exposed to chronic restraint stress, without changes in spine density.
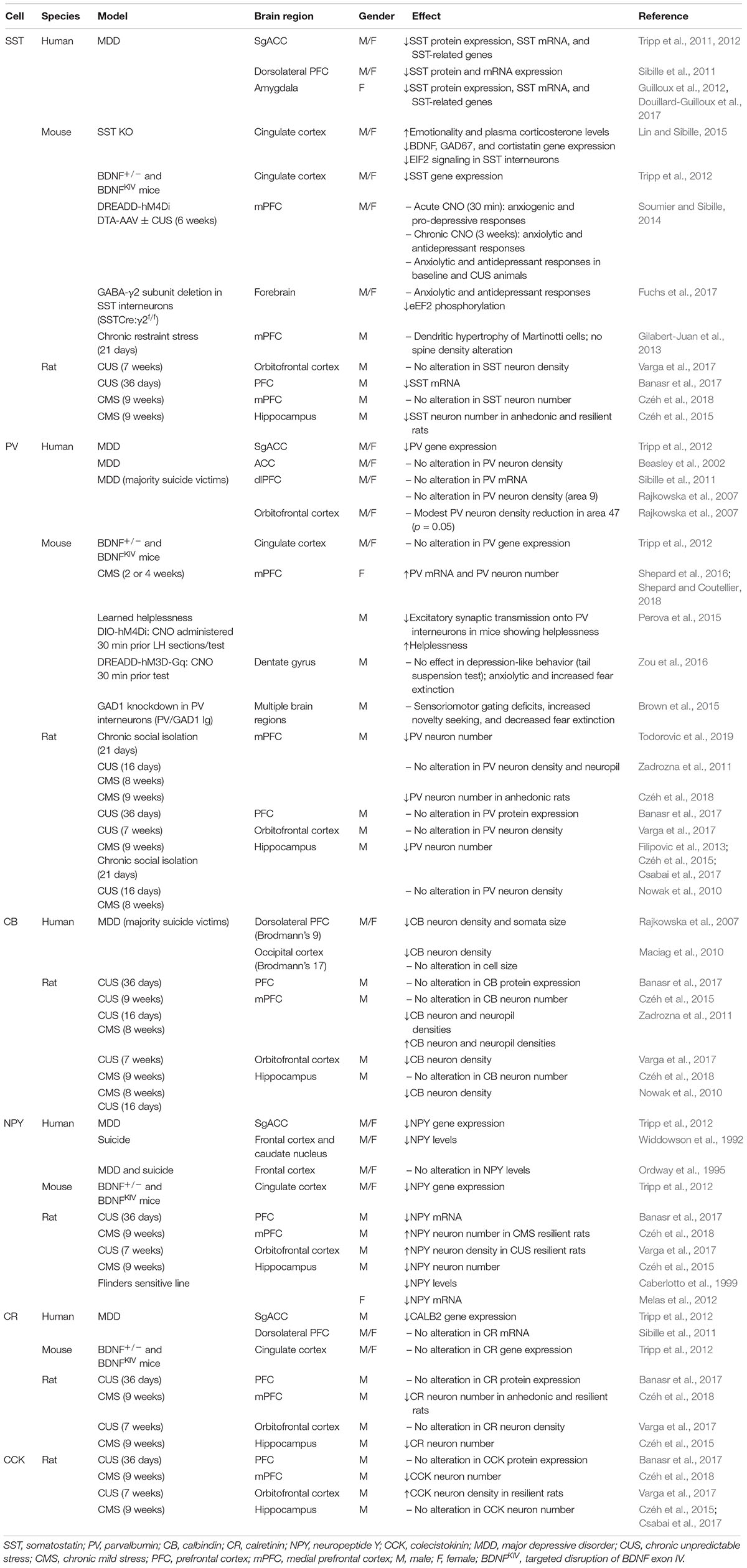
Table 1. Studies of GABAergic interneuron subtypes in MDD and animal models of depression.
The association between SST interneurons and the pathophysiology of MDD has been more directly supported through pharmacological and genetic manipulations in rodents. Mice lacking SST (SST-KO) exhibited increased anxiety- and depressive-like behaviors; elevated basal plasma corticosterone; and reduced BDNF, GAD67, and cortistatin genes expression ( Lin and Sibille, 2015 ). Disinhibition of SST interneurons by deletion of GABA A -containing γ2 subunit in SST neurons (SSTCre:γ2 f/f ) resulted in enhanced inhibitory input to pyramidal cells in the hippocampus and cingulate cortex, and consequently produced anxiolytic- and antidepressant-like phenotypes ( Fuchs et al., 2017 ). Interestingly, acute chemogenetic-induced inhibition of SST interneurons in the mPFC promoted anxiety and depressive-like responses, whereas chronic silencing or chemical ablation had the opposite effect ( Soumier and Sibille, 2014 ). Furthermore, mice with constitutive, heterozygous deletion of the BDNF gene (BDNF+/-) or with targeted disruption of exon IV (BDNF KIV ), causing a reduction or blockade of activity-dependent BDNF expression and depressive-like behaviors, showed reduced SST and NPY gene expression in the cingulate cortex ( Tripp et al., 2012 ).
Somatostatin co-localizes with calbindin and NPY, and these neuropeptides have also been implicated in mood disorders. Reductions in calbindin and NPY markers were found in the frontal cortex of MDD patients ( Widdowson et al., 1992 ; Rajkowska et al., 2007 ; Maciag et al., 2010 ; Tripp et al., 2011 , 2012 ), as well as in the PFC and hippocampus of rats submitted to different rodent models of depression, including CUS, BDNF mutant mice, or the Flinders sensitive line of rat ( Caberlotto et al., 1999 ; Nowak et al., 2010 ; Zadrozna et al., 2011 ; Melas et al., 2012 ; Tripp et al., 2012 ; Czéh et al., 2015 , 2018 ; Banasr et al., 2017 ; Varga et al., 2017 ). However, other studies failed to detect statistical differences in chronic stress models ( Czéh et al., 2015 , 2018 ; Banasr et al., 2017 ). Additionally, NPY neuronal density was increased in the orbitofrontal cortex and mPFC (IL) of rats that were considered resilient to CUS behavioral effects, but unchanged in anhedonic animals ( Varga et al., 2017 ; Czéh et al., 2018 ). In depressed patients, the levels of NPY in the CSF or plasma were inversely correlated with anxiety symptoms and with attempted suicide ( Widerlov et al., 1988 ; Westrin et al., 1999 ). Other markers, such as CCK and calretinin, seem not to be consistently affected in stress models or MDD ( Sibille et al., 2011 ; Zadrozna et al., 2011 ; Tripp et al., 2012 ; Banasr et al., 2017 ; Csabai et al., 2017 ), notwithstanding a study reporting decreased immunoreactivity of these peptides in the mPFC of rats submitted to CMS ( Czéh et al., 2018 ).
Although several studies have failed to detect robust differences in the expression of PV in rodent models and MDD subjects ( Beasley et al., 2002 ; Cotter et al., 2002 ; Rajkowska et al., 2007 ; Nowak et al., 2010 ; Sibille et al., 2011 ; Zadrozna et al., 2011 ; Banasr et al., 2017 ), there are reports that PV interneurons contribute to regulation of E:I within the PFC that influences emotional responses ( Perova et al., 2015 ; Ferguson and Gao, 2018 ). One study has reported a decrease in PV gene expression in SgACC post-mortem tissues of depressed patients ( Tripp et al., 2012 ), and a modest reduction in PV immunoreactivity was found in the orbitofrontal cortex ( p = 0.05, Brodmann area 47) ( Rajkowska et al., 2007 ). In rodents, CMS caused a reduction in PV neuron number in the mPFC of anhedonic rats, whereas SST density was unchanged ( Czéh et al., 2018 ). A decrease in PV cell number was also found in rats submitted to chronic social isolation ( Todorovic et al., 2019 ). By contrast, there was a report of increased PV expression in the PFC of female mice after 2 weeks of CUS ( Shepard et al., 2016 ; Shepard and Coutellier, 2018 ). Also, mice that showed helplessness behavior in response to inescapable stress exposure showed a reduction in excitatory synaptic transmission onto PV interneurons in the mPFC, and selective chemogenetic inactivation of PV cells further increased helplessness responses ( Perova et al., 2015 ).
However, other genetic approaches have reported complex behavioral changes. Knockdown of the Gad1 transcript specifically in PV interneurons ( Pvalb / Gad1 Tg) produced a decrease in PV-induced GABAergic activity in multiple brain regions, leading to sensoriomotor gating deficits, increased novelty seeking, and decreased fear expression ( Brown et al., 2015 ). Besides the PFC, PV cells located in the hippocampus also play an important role in the modulation of affective behaviors as well as memory. Acute activation of PV interneurons in the dentate gyrus of the hippocampus through DREADD-hM3D-Gq virus and CNO administration (30 min) did not affect depressive-like behavior in the tail suspension test, but produced anxiolytic-like responses and increased fear extinction ( Zou et al., 2016 ). Also, CMS or chronic social isolation induced a decrease in PV-immunoreactive cells in the hippocampus, whereas CCK and calbindin expression remained unchanged ( Filipovic et al., 2013 ; Czéh et al., 2015 ; Csabai et al., 2017 ). Thus, divergence in the responses observed among these studies in the literature highly are related to the brain region studied, the experimental protocol (timing, stress paradigms), and sex differences. Also, it is noteworthy that PV interneurons are under inhibitory control of other interneuron populations, such as SST, making the resultant responses even more complex.
Taken together, the studies mentioned highlight the complexity of segregating the GABAergic system into different subclasses of interneurons to study the pathophysiology of depression, but demonstrate the importance of understanding how these cells locally interact and integrate diverse neurocircuits that control affective behavioral responses.
The GABAergic System as a Therapeutic Target for the Treatment of MDD
Although extensive efforts have been conducted to develop new therapeutic interventions, the current pharmacological treatment approaches still recommend the use of SSRIs as first-line medications for the treatment of MDD. These drugs, along with other classic antidepressants, such as tricyclics and monoamine oxidase inhibitors, primarily facilitate monoaminergic systems, including 5-HT and norepinephrine. However, the emergence of fast-acting antidepressants, notably ketamine, provide evience for other neurotransmitter systems for the treatment, as well as pathophysiology of MDD. Evidence that normalization of GABA-mediated E:I imbalance in the PFC is a shared mechanism of action between different classes of antidepressants, providing further support for the involvement of GABAergic dysfunction in the etiology of MDD. In this section, we will review the literature showing how first-line monoaminergic antidepressants and rapid-acting agents can influence the GABAergic system. We will discuss how fast-acting antidepressants provide a new understanding of the pathophysiology of depression, leading to connections between the glutamatergic, GABAergic, and neurotrophic hypotheses of depression. Finally, we will discuss the antidepressant potential of agonists, antagonists, or allosteric modulators of GABA A and GABA B receptors, as well as neuropeptides that target specific subpopulations of GABA interneurons and cannabinoid agents.
Classic Monoaminergic Antidepressants: Effects Beyond Monoamines
The correlation between GABA deficits in the brain, stress, and MDD became more evident with investigations showing that SSRIs, electroconvulsive therapy, and transcranial magnetic stimulation normalize the reduction in cortical and plasmatic GABA levels, as well as in GAD67 expression in MDD subjects and rodents subjected to chronic stress ( Sanacora et al., 1999 , 2004 ; Bhagwagar et al., 2004 ; Goren et al., 2007 ; Kucukibrahimoglu et al., 2009 ; Karolewicz et al., 2010 ; Dubin et al., 2016 ). Besides decreased GABA levels, MDD patients and chronically stressed animals have reduced levels of allopregnanolone (brain and plasma), an endogenous neurosteroid that acts as a GABA A receptor positive allosteric modulator (discussed in more detail below). This deficit was reversed by chronic administration of SSRIs such as fluoxetine ( Uzunov et al., 1996 ; Romeo et al., 1998 ; Uzunova et al., 1998 , 2004 ; Strohle et al., 1999 ; Dong et al., 2001 ; Guidotti et al., 2001 ; Pinna et al., 2006 , 2009 ); interestingly, in vitro evidence suggests that SSRIs can directly interact with the enzymes involved in neurosteroid synthesis ( Griffin and Mellon, 1999 ). Chronic treatment with the classic monoaminergic antidepressant desipramine, but not fluoxetine, also normalized the elevated serum corticosterone levels and the pro-depressive behaviors of γ2+/- mice ( Shen et al., 2010 ). In this same study, subchronic treatment with desipramine had no effect ( Shen et al., 2010 ), suggesting that this drug acts over time to balance GABAergic inhibition deficits. In another study, chronic fluoxetine treatment induced pro-depressive and anxiogenic-like effects in γ2+/- mice ( Benham et al., 2017 ), pointing to a requirement of GABA A -containing γ2 subunit in the antidepressant effect of SSRIs. Interestingly, studies reported that fluoxetine can act directly as an allosteric modulator of GABA A receptors ( Robinson et al., 2003 ).
Indeed, direct interactions between the GABAergic and serotoninergic systems in the raphe nucleus and cortical regions have been reported ( Celada et al., 2001 ; Puig et al., 2004 ; Santana et al., 2004 ; Llado-Pelfort et al., 2012 ). In the PFC, both pyramidal glutamatergic neurons and GABAergic interneurons, notably PV positive cells, express serotoninergic receptors (mainly 5HT 1A and 5HT 2A ) ( Santana et al., 2004 ; Celada et al., 2013 ). 5HT 1A receptor agonists, such as 8-OH-DPAT, have a preferential action on GABA interneurons, resulting in pyramidal neuron disinhibition and enhancement of cell firing in PFC and targeted subcortical structures, such as the ventral tegmental area ( Llado-Pelfort et al., 2012 ). On the other hand, the excitability of pyramidal neurons in the mPFC can be inhibited by activation of GABA interneurons through 5HT 3 receptors ( Puig et al., 2004 ). In this regard, multimodal drugs that are high affinity 5HT 3 receptor antagonists, such as vortioxetine, show antidepressant efficacy in clinical studies and have been used as atypical antidepressants to treat MDD ( Thase et al., 2016 ; Artigas et al., 2018 ). Moreover, the majority of serotoninergic cell bodies in the raphe nucleus express GABA B receptors, which control serotoninergic cell firing as well as the release of monoamines in other brain regions ( Bowery et al., 1980 ; Abellan et al., 2000 ; Serrats et al., 2003 ). The antidepressant-like effects of the GABA B antagonist CGP56433A were abolished by prior treatment with a tryptophan hydroxylase inhibitor, which depletes serotonin levels ( Slattery et al., 2005 ). Additionally, several different monoaminergic antidepressants increase GABA B receptor binding and function in the rat frontal cortex ( Lloyd et al., 1985 ; Gray and Green, 1987 ).
Fast-Acting Glutamatergic Antidepressants: Is It All Glutamate?
In recent years, the mechanisms underlying the actions of ketamine have been extensively studied because of its rapid (within hours), sustained (up to 7 days), and efficacious effects (effective in patients considered treatment resistant) ( Berman et al., 2000 ; Zarate et al., 2006 ). Related agents, including ketamine stereioisomers and metabolites, have also demonsrated rapid effects in rodent models. These drugs share the ability to influence, directly or indirectly the enhancement of glutamatergic signaling in the brain, promoting post-synaptic AMPA-mediated calcium influx that leads to BDNF release by pyramidal neurons ( Lepack et al., 2014 , 2016 ; Zhou et al., 2014 ). Extracellular BDNF, in turn, activates TrkB receptors in the membrane, resulting in stimulation of intracellular signaling cascades, including Akt, eukaryotic elongation factor 2 kinase (eEF2K), and mTORC1 that results in synaptic actions that contribute to antidepressant behavioral responses ( Li et al., 2010 ; Autry et al., 2011 ; Duman et al., 2016 ).
The molecular and cellular mechanisms underlying the rapid enhancement of glutamatergic signaling in the PFC by ketamine have been of particular interest. One hypothesis is that ketamine first targets NMDA receptors specifically located in cortical interneurons, notably, SST and PV subtypes. Because these GABA inhibitory neurons are tonic firing they would be more sensitive to antagonist blockade as tonic activity would remove the Mg 2+ block of the NMDA receptor allowing ketamine to enter the channel pore and block further activation of Ca 2+ entry ( Fee et al., 2017 ; Ghosal et al., 2017 ). Blockade of GABAergic interneuron firing would thereby decrease GABA release, resulting in disinhibition of excitatory pyramidal neurons and subsequently produce a glutamate burst that could drive activity dependent synaptic plasticity ( Duman et al., 2016 ). An alternative hypothesis is that ketamine acts directly on pyramidal neurons to block resting state NMDA receptor activity driven by spontaneous glutamate release that produces synaptic changes via deactivation of eEF2K, resulting in increased synthesis of synaptic proteins ( Autry et al., 2011 ). These two theories may not be mutually exclusive, although it is difficult to explain how NMDA receptors would be at resting levels in the presence of a known glutamate burst ( Moghaddam et al., 1997 ). In either case, there is an increase in synaptic protein synthesis that underlies long-lasting changes (approximately 1 week) that correspond to the time course for the antidepressant behavioral actions of ketamine. It is also possible that more long-lasting ketamine metabolites contribute to the sustained actions of ketamine ( Zanos et al., 2016 ; Fukumoto et al., 2019 ). The glutamate burst produced by ketamine appears to be contradictory with evidence of elevated glutamate levels in the brains of MDD subjects ( Stone et al., 2012 ), although other studies have reported no significant differences ( Valentine et al., 2011 ; Taylor et al., 2012 ). However, it is important to note that the ketamine-induced burst of glutamate is transient, lasting approximately 1 h, and then levels return to control ( Moghaddam et al., 1997 ). Although transient, the glutamate burst results in activity-dependent synaptic changes that are long-lasting.
Although much of the current work has focused on glutamate synaptic changes in the actions of ketamine, there is also increasing evidence that GABA alterations contribute to the ketamine response, by reestablishing E:I balance in the PFC via homeostatic self-tuning adaptations. This local reorganization could influence microcircuits in target regions by reestablishing firing patterns, and thereby promoting antidepressant effects. This idea is supported by recent evidence that the fast antidepressant effects of ketamine are accompanied by a robust increase in GABA levels in the mPFC of MDD patients ( Milak et al., 2016 ) and in the ACC of rats subjected to CUS ( Perrine et al., 2014 ), although another study failed to detect differences in the occipital cortex ( Valentine et al., 2011 ). One possibility for these discrepancies, in addition to the different cortical subregions analyzed, is the timepoint at which MRS data were collected. Whereas one study was conducted during ketamine infusion ( Milak et al., 2016 ), the other was carried out after ketamine ( Valentine et al., 2011 ); by the end of the infusion, it was shown in the former study that the increase in amino acid responses was no longer detectable ( Milak et al., 2016 ).
Also, a SPECT study reports that S-ketamine administration leads to alterations of GABA A receptor binding in the dorsomedial PFC of healthy subjects ( Heinzel et al., 2008 ). Likewise, studies in cultured murine neurons provide evidence that ketamine increases the activity of extrasynaptic GABA A receptors in the cortex and hippocampus ( Wang et al., 2017 ). Combined administration of sub-effective doses of muscimol, a potent and selective agonist of GABA A receptors, and ketamine, produced antidepressant-like effects in female mice ( Rosa et al., 2016 ). In this same study, the antidepressant effects of ketamine were blocked by the GABA B agonist baclofen, suggesting that the antidepressant actions of ketamine could involve activation of GABA A and blockade of GABA B receptors ( Rosa et al., 2016 ). In support of glutamatergic and GABAergic interactions in ketamine responses is data showing that a single dose of the ketamine induced antidepressant-like effects and normalized the glutamatergic deficits, including reduced cell surface NMDA and AMPA receptor levels and impaired synaptic function in the hippocampus and mPFC of γ2+/- mice ( Ren et al., 2016 ). Moreover, ketamine potentiated pre- and post-synaptic GABAergic synapses selectively in the ACC of these animals ( Ren et al., 2016 ). In addition, we have found that a single dose of ketamine increases markers of GABA in the PFC, including increased levels of VGAT, GAD, and gephyrin ( Ghosal et al., 2018 , SfN abstract). Thus, although more studies are needed to clarify how ketamine modulates the GABAergic system, the current evidence indicates that ketamine enhances GABA levels/function in the brain as well as GABA A receptors activity.
In addition, it was reported that fast-acting agents, such as Ro-25-6981, a GluN2B-selective NMDA receptor antagonist, induce antidepressant effects by promoting GABA B receptor surface expression and increasing postsynaptic GABA B -mediated resting L -type calcium channel activity, resulting in an increased intracellular calcium, recruitment of BDNF/mTORC1 pathways, and protein synthesis ( Workman et al., 2013 , 2015 ). Accordingly, MDD and suicide patients have decreased levels of blood and brain BDNF levels and transcripts ( Dwivedi et al., 2003 ; Shimizu et al., 2003 ; Kim et al., 2007 ; Guilloux et al., 2012 ; Banerjee et al., 2013 ); reduced BDNF levels in blood were absent in patients taking antidepressants ( Shimizu et al., 2003 ). However, while monoaminergic antidepressants take weeks to modulate neurotrophic factor expression, the rapid elevation in BDNF “release” and signaling by ketamine is shared by other fast-acting agents, such as the non-selective muscarinic receptor antagonist scopolamine, the NMDA receptor modulator GLYX-13 (rapastinel), the ketamine metabolite (2R,6R)-Hydroxynorketamine [(2R,6R)-HNK], and the mGlu2/3 receptor antagonist LY341495 ( Liu et al., 2012 ; Lepack et al., 2016 ; Ghosal et al., 2018 ; Kato et al., 2018 ) and may explain, at least in part, the fast versus slow response rates of these agents. Moreover, in vitro and in vivo studies suggest that BDNF induces antidepressant-like effects via increased phosphorylation of γ2 subunit, resulting in an increase of GABA A receptor accumulation and stability in the cell surface, and in an enhancement of synaptic inhibition efficacy in the hippocampus and PFC ( Jovanovic et al., 2004 ; Vithlani et al., 2013 ). Thus, the upregulation of GABA B receptors induced by NMDA receptor blockade and consequent activation of BDNF/mTORC1 signaling, as well as the role of BDNF on GABA A receptors phosphorylation and enhancement of GABAergic mIPSC amplitude and frequency, could be a link associating the GABA/glutamate balance deficits to the neurotrophic theory of depression ( Figure 1 ).
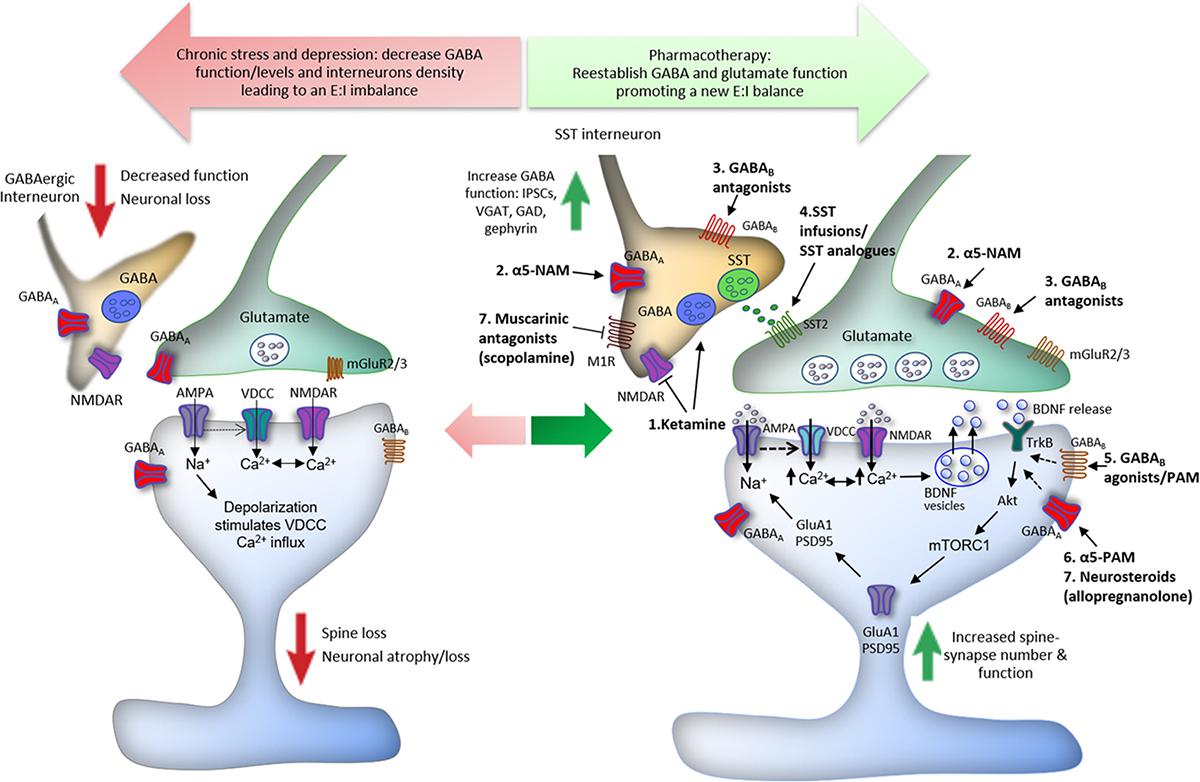
Figure 1. Proposed mechanisms underlying the action of ketamine and GABA-related drugs in the reestablishment of cortical excitatory–inhibitory (E:I) balance. Chronic stress induces spine loss and dendritic atrophy in pyramidal glutamatergic cells, and decreased GABAergic interneuron markers, leading to a reduction in the levels and function of GABA in the PFC. GABAergic dysfunction disturbs the optimal E:I balance in the brain and compromises the integrity of neurocircuits, contributing to the development of major depressive disorder (MDD) and other stress-related disorders. The E:I imbalance can be reversed by drugs via different GABA-related mechanisms. (1) Low doses of ketamine induce a glutamate burst in the PFC via blockade of NMDA receptors located in GABAergic interneurons; the tonic firing of these interneurons, notably parvalbumin (PV) and somatostatin (SST), is driven by NMDA receptors that are more sensitive to ketamine because of activity dependent of the Mg 2+ block. This leads to disinhibition of pyramidal neurons causing activation of post-synaptic AMPA receptors; this in turn induces neuronal depolarization and activation of voltage-dependent Ca 2+ channels (VDCCs). The enhancement of intracellular Ca 2+ influx leads to BDNF release and stimulation of TrkB receptors, which activates mTORC1 signaling inducing protein synthesis required for the formation of new spines and synaptic plasticity. Ketamine also facilitates GABA-mediated effects, increasing IPSCs, VGAT, GAD, and gephyrin in the PFC, reversing the GABA deficits caused by chronic stress exposure. (2) Likewise, α5-GABA A negative allosteric modulators (α5-NAMs) and (3) GABA B receptors antagonists, probably located in GABAergic interneurons, enhance glutamatergic neurotransmission and produce ketamine-like effects. (4) Infusions of SST or SST analogs into limbic brain regions produce antidepressant-like effects through activation of SST2 receptors. (5) Finally, activation of post-synaptic GABA B receptors by agonists or positive allosteric modulators (PAMs), as well as activation of α5-GABA A by PAMs, and other GABA A subunits by neurosteroids, notably allopregnanolone (6), can also recruit BDNF expression and signaling that could contribute to antidepressant responses (dashed arrows).
GABA Ligands
Gaba a -α2 positive allosteric modulators.
Deletion studies of α2-containing GABA A subunit demonstrate a role of these receptors in depressive behaviors and suggest that agonists or positive modulators could produce antidepressant effects. Benzodiazepines (BZD), GABA A positive allosteric modulators developed in the 1950s, are one of the most widely used thereapeutic agents for the treatment of psychiatric disorders, due to anxiolytic actions at the GABA A -α2 receptor and hypnotic effects at the GABA A -α1 ( Mohler et al., 2002 ). However, the efficacy of classical BZD monotherapy for the treatment of MDD has not been consistently reported, in part due to methodological confounds (i.e., small sample size, variable duration of treatment, and cotreatment with antidepressant agents), as well as high comorbidity with anxiety disorders ( Pehrson and Sanchez, 2015 ). However, the triazolobenzodiazepine alprazolam was shown to induce significant antidepressant effects similar to tricyclic drugs in several meta-analysis studies ( Jonas and Cohon, 1993 ; Birkenhager et al., 1995 ; Petty et al., 1995 ; van Marwijk et al., 2012 ). This antidepressant potential has been attributed to its differential chemical structure formed by a triazol ring fused to the diazepine ring. Recently, selective agonists or positive modulators of GABA A -containing α2/α3 subunits, such as TPA023 and eszopiclone, have been developed and are proposed as potential antidepressants ( Atack, 2011 ; Atack et al., 2011 ; Vollenweider et al., 2011 ). When co-administered with SSRIs, eszopiclone, a preferential α2/α3-GABA A positive modulator, induced a faster onset of efficacy and greater treatment response, suggesting a synergistic effect ( Fava et al., 2006 , 2011 ; Krystal et al., 2007 ). Although it is still unclear if increasing the activity of GABA A receptor is effective in relieving depression symptoms, given that some BZD seem to have greater antidepressant efficacy (i.e., alprazolam versus diazepam), a more thorough understanding of the role of different subunits/subtypes of GABA A receptors could result in the development of more selective and efficacious antidepressant drugs.
GABA A -α5 Negative Allosteric Modulators
Recently, a new class of fast-acting antidepressants that specifically target the GABA A -containing α5 subunit has emerged ( Atack et al., 2009 ; Zanos et al., 2017 ; Xiong et al., 2018 ). These receptors were shown to be up-regulated in the cortex and hippocampus of depressed patients and stressed mice ( Matsumoto et al., 2007 ; Xiong et al., 2018 ). Interestingly, preclinical studies have demonstrated that both positive and negative allosteric modulators of GABA A -α5 receptors produce rapid antidepressant-like effects or prevent the behavioral responses induced by chronic stress ( Zanos et al., 2017 ; Xiong et al., 2018 ). This apparent discrepancy could be due to ketamine-like induction of a glutamate burst for negative modulators and GABA A receptor dependent effects of positive modulators. In one study, MRK-016, a negative allosteric modulator of GABA A -α5 receptors and partial inverse agonist of the BZD-binding site, produced a transient increase in electroencephalogram γ power, similar to ketamine. These effects of MRK-016 were abolished by NBQX, an AMPA-type glutamate receptor antagonist, suggesting a fast recruitment of the glutamatergic system and activity-dependent effects. Importantly, perhaps because of the restricted distribution of GABA A -α5 receptors in the brain (mainly in the cortex and hippocampus), MRK-016 did not induce the typical ketamine-like side effects indicative of psychotomimetic or cognitive impairment ( Zanos et al., 2017 ). Unfortunately, further development of this compound has been discontinued because of low tolerability in elderly subjects ( Atack et al., 2009 ; Atack, 2011 ; Rudolph and Knoflach, 2011 ). Another GABA A -α5 negative allosteric modulator, L-655,708, also restored alterations in hedonic behaviors induced by chronic stress and the excitatory synaptic strength in the CA1 region of the hippocampus ( Fischell et al., 2015 ). A single dose of L-655,708 increased the expression of the GluA1 subunit of the AMPA receptor, suggesting that, similar to MRK-016, it produces an indirect potentiation of excitatory synapses. Other GABA A -α5 negative modulators have been tested as cognitive enhancers in clinical trials, but these agents have not been tested for effectiveness in depression ( Rudolph and Knoflach, 2011 ).
Surprisingly, similar antidepressant-like effects were found after acute and chronic enhancement of α5-GABA A activity by a positive modulator; however, this response was restricted to female mice and absent in males, suggesting sex-effects ( Piantadosi et al., 2016 ). Since the behavioral sex differences could not be explained by differential pharmacokinetic effects (i.e., different brain concentrations), it is possible that α5-GABA A positive modulators interact with steroid hormones to produce an antidepressant response. Indeed, GABA A receptor subunits, such as δ, are highly sensitive to neurosteroids (see next section) and are differentially modulated across the estrous cycle ( Maggi and Perez, 1986 ; Maguire and Mody, 2007 ).
Neurosteroids
Endogenous neuroactive ligands synthetized from progesterone, deoxycorticosterone, or testosterone, referred to as neurosteroids interact with a number of targets, most notably GABA A receptors and act as positive or negative allosteric modulators. Numerous preclinical evidence demonstrate that neurosteroids modulate the HPA axis and adaptive responses to stress exposure ( Crowley and Girdler, 2014 ), and exert anxiolytic or antidepressant effects in rodent models ( Khisti et al., 2000 ; Guidotti et al., 2001 ; Rodriguez-Landa et al., 2007 ). Specifically, the progesterone-derived neurosteroids, allopregnanolone, a potent positive allosteric modulator of both synaptic and extrasynaptic GABA A receptors, were shown to rapidly modulate BDNF expression in the rat brain ( Naert et al., 2007 ; Nin et al., 2011 ; Almeida et al., 2019 ), which could explain its fast onset for antidepressant responses. Allopregnanolone has been tested for the treatment of post-partum depression using a formulation developed by SAGE, referred to as brexanolone. The rationale for this study is based on the precipitous drop at the time of delivery of estrogen and progesterone, and consequently a drop in allopregnanolone resulting in a loss of this key positive allosteric modulator of GABA A receptors and a withdrawal like effect. Brexanolone has been delivered intravenously and tested in two Phase II and Phase III trials. Due to its very promising results, it was recently granted a FDA Breakthrough Therapy Designation for the treatment of post-partum depression, and it has also been tested in placebo-controlled Phase III trials for the treatment of MDD ( Kanes S. et al., 2017 ; Kanes S.J. et al., 2017 ; Meltzer-Brody et al., 2018 ; Wilkinson and Sanacora, 2018 ). Another compound, SAGE-217, an improved allopregnanolone formula with higher oral bioavailability and longer half-life, which can be used for once daily oral administration, successfully completed a Phase II study for MDD and also received a FDA Breakthrough Therapy Designation ( Sage Therapeutics, 2017 ). In addition, ganaxolone (Marinus Pharmaceuticals), a neuroactive steroid that acts as a GABA A positive allosteric modulator, was initially developed for the treatment of epilepsy and anxiety, and currently is under Phase II trials for post-partum depression ( Wilkinson and Sanacora, 2018 ).
GABA B Receptors Ligands
The first prototypical GABA B receptor agonist, bacoflen, was synthetized in 1962 and it was an invaluable pharmacological tool that influenced studies that led to the characterization of GABA B receptors in the 1980s. Years later, with the development of the first GABA B receptor antagonists, phacoflen and saclofen, additional work has lead to the development of compounds that more specifically target GABA B receptors. Given that the GABA B receptor is a heterodimer of two subunits (GABA B1 and GABA B2 ), that GABA B1 has been reported to have several splice variants (mainly GABA B1A and GABA B1B ), and that GABA B receptors are located both pre- and post-synaptically, pharmacological studies targeting these receptors report very challenging and complex results ( Bowery et al., 1980 , 1981 ; Kaupmann et al., 1997 ; Cryan and Kaupmann, 2005 ; Jacobson et al., 2018 ).
Preclinical studies suggest that GABA B agonists, positive allosteric modulators, and antagonists can produce antidepressant effects; unfortunately, there are very few clinical studies due to the lack of compounds adequate for human testing ( Alexander, 2017 ). In rats, acute administration (i.p.) of baclofen or SKF97541, both GABA B receptors agonists, or CGP7930, a GABA B positive allosteric modulator, induced antidepressant-like effects in the forced swim test, whereas chronic administration increased the escape failures in the learned helplessness test ( Nakagawa et al., 1999 ; Frankowska et al., 2007 ). However, other studies failed to find significant effects for agonists or positive allosteric modulators ( Nakagawa et al., 1999 ; Slattery et al., 2005 ; Nowak et al., 2006 ). In humans, one study reported that bacoflen intensified depressive symptoms in MDD patients ( Post et al., 1991 ). Studies of GABA B antagonists have yielded more consistent results, with a large range of studies showing antidepressant-like effects induced by several different compounds administered either acute- or chronically, such as CGP36742 (also known as SGS742), CGP51176, CGP51176A, CGP56433A, and SCH50911 ( Bittiger et al., 1993 ; Nakagawa et al., 1999 ; Mombereau et al., 2004 ; Slattery et al., 2005 ; Nowak et al., 2006 ; Frankowska et al., 2007 ). Interestingly, CGP36742 decreased learned helplessness behavior in rats ( Nakagawa et al., 1999 ) and increased BDNF and NGF release in the cortex and hippocampus ( Heese et al., 2000 ), as well as increased extracellular glutamate and SST in the rat hippocampus ( Nyitrai et al., 1999 ; Nyitrai et al., 2003 ). Notably, this is the first GABA B receptor antagonist that underwent clinical trials for cognition-enhancing activity and improved attention in patients with mild cognitive impairment ( Froestl et al., 2004 ). Thus, considering that: (i) MDD patients in general have an upregulation of GABA B receptors; (ii) genetic deletion of GABA B receptors produce antidepressant-like effects; (iii) GABA B receptors are implicated in the antidepressant actions of fast agents such as ketamine ( Workman et al., 2013 , 2015 ; Rosa et al., 2016 ); and (iv) GABA B receptors antagonists offer a promising strategy for the development of novel fast-acting antidepressants, more studies and clinical trials are warranted to identify effective and safe agents.
Neuropeptides
Because of the postmortem evidence of selective alterations of GABA interneuron subytpes, it is interesting to speculate on approaches to target the function of specific subpopulations of interneurons based on expression of selective neuropeptides. Preclinical studies demonstrate promising pharmacological evidence for two neuropeptides, NPY and SST, to treat MDD. Intraperitoneal or direct intracerebral (lateral ventricle, hippocampus, amydgala, or septum) infusions of the SST peptide or small molecule SST agonists induce anxiolytic- and antidepressant-like effects in naïve and chronically stressed rodents, as well as exert inhibitory feedback on the HPA axis ( Engin et al., 2008 ; Engin and Treit, 2009 ; Yeung et al., 2011 ; Prevot et al., 2017 ). There are five (1–5) SST Gi-protein-coupled receptors that are distributed on SST-expressing GABAergic interneurons, and are mainly coupled with induction of K+ conductance leading to neuronal hyperpolarization ( Jiang et al., 2003 ; Meis et al., 2005 ). The development of selective SST compounds and genetic approaches using specific SST receptor subtypes knockout animals suggest that SST2 receptor, the most abundant subtype in the brain, is a key target receptor for the antidepressant effects of SST ( Viollet et al., 2000 ; Engin and Treit, 2009 ; Prevot et al., 2017 ). However, the plasma half-life of SST is very short making it unsuitable for clinical trials ( Pinter et al., 2006 ; Engin and Treit, 2009 ).
Two more stable analogs, octreotide and lanreotide, have been tested in clinical studies to treat a wide range of diseases, such as inflammation, tumor growth, and pain ( De Jong et al., 1999 ; Hofland et al., 1992 ; Carlton et al., 2004 ; Pinter et al., 2006 ); although these drugs show a high affinity to SST2 receptors, they lack selectivity, and induce a broad spectrum of undesired effects in both periphery and CNS ( Pawlikowski and Melen-Mucha, 2003 ). Drugs that act as selective SST2 receptor agonists, such as L-779,976, have never been tested in clinical trials. Thus, given that SST levels were reported to be lower in the brain of MDD patients and stressed rodents ( Frye et al., 2003 ; Tripp et al., 2011 ), which can be normalized by monoaminergic drugs ( Faron-Gorecka et al., 2016 , 2018 ), and preclinical evidence that SST induces antidepressant-like effects, clinical studies testing the antidepressant potential of selective SST2 receptor analogs with longer half-life merit additional attention.
Early preclinical studies also provided evidence that central administration of NPY induces anxiolytic- and antidepressant-like effects ( Heilig and Murison, 1987 ; Heilig et al., 1989 , 1992 ; Pich et al., 1993 ; Broqua et al., 1995 ; Redrobe et al., 2002a , b ), as well as promotes stress adaptation and resilience ( Thorsell et al., 2000 ; Sajdyk et al., 2008 ; Yang et al., 2018 ). In the brain, at least four subtypes of Gi-coupled receptors for NPY were identified (Y1, Y2, Y4, and Y5) ( Larhammar and Salaneck, 2004 ) and the antidepressant-like effects of NYP are suggested to be mediated by Y1R ( Redrobe et al., 2002a ; Karlsson et al., 2008 ). Interestingly, NPY levels were decreased in treatment-resistant MDD patients ( Heilig et al., 2004 ) and increased after treatment with SSRIs, an effect that was inversely correlated to depression severity ( Nikisch et al., 2005 ). NPY administration in humans also represents a challenge due to short half-life, as well as undesired effects. To overcome this problem, clinical studies have focused on the therapeutic potential of intranasal NPY administration ( Lacroix and Mosimann, 1996 ; Lacroix et al., 1996 ; Hallschmid et al., 2004 , 2003 ). While a recent randomized dose-ranging study found that intranasal NPY is effective for the treatment of posttraumatic stress disorder with reduced side effects ( Sayed et al., 2018 ), the antidepressant efficacy of intranasal NPY in MDD patients has not been tested. Given that Y1 agonists or Y2 antagonists also show promise in preclinical studies as antidepressants ( Redrobe et al., 2002b ), further studies of selective drugs as well as intranasal administration of NPY in MDD patients are warranted.
Cannabinoid Agents
Endocannabinoids, such as anandamide and 2-arachidonoylglycerol, are pivotal endogenous neuromodulators that control GABA and glutamate release in the brain, mainly through actions on cannabinoid type 1 (CB 1 ) and cannabinoid type 2 (CB 2 ) receptors (although some endocannabinoids can also activate transient receptor potential vanilloid type 1 receptors) ( Fogaça et al., 2012 ). CB 1 and CB 2 are G i/o -coupled receptors mostly located pre-synaptically, and their activation results in hyperpolarization and reduction of neurotransmitter release ( Szabo and Schlicker, 2005 ). In the neocortex, CB 1 receptors are expressed by multiple interneuron subpopulations, mostly in CCK-, but are also found in SST-, calbindin-, and VIP-expressing cells, and at lower levels in glutamatergic neurons ( Hill et al., 2007 ; Wedzony and Chocyk, 2009 ). Given that (i) CB 1 receptors are highly expressed in cortical and limbic regions ( Pettit et al., 1998 ; Wang et al., 2003 ), (ii) CB 1 receptors are expressed in cortical interneurons and glutamatergic pyramidal cells, thereby modulating both GABA and glutamate release ( Hill et al., 2007 ), and (iii) endocannabinoids act as retrograde messengers to mediate depolarization-induced suppression of E (DSE) and I (DSI) ( Diana and Marty, 2004 ; Hill et al., 2007 ), it is not surprising that the endocannabinoid system plays an important role in orchestrating cortical E:I balance and controlling stress responses. Indeed, in the mPFC, endocannabinoids contribute to the termination of HPA activity during stress responses through inhibition of GABA release, increasing the outflow of principal interneurons to target regions ( Hill et al., 2011 ).
Cannabinoid agents have shown promise for the treatment of anxiety disorders and depression ( Poleszak et al., 2018 ; Stampanoni Bassi et al., 2018 ). The most studied compound for therapeutic use is cannabidiol (CBD), the major non-psychotomimetic substance from Cannabis sativa. Although CBD has a low affinity for CB 1 and CB 2 receptors, it enhances endocannabinoid neurotransmission by interfering with the function of fatty acid amide hydrolase (FAAH), the enzyme responsible for anandamide degradation ( Bisogno et al., 2001 ; De Petrocellis et al., 2011 ; Fogaça et al., 2018 ). Also, CBD acts as an allosteric modulator of 5HT 1A receptors and was recently shown to exert direct actions at GABA A receptors ( Russo et al., 2005 ; Bakas et al., 2017 ). Accumulating clinical and pre-clinical evidence suggests that acute and chronic administration of CBD induces anxiolytic and antidepressant effects, as well as prevents the behavioral consequences of CUS ( Schiavon et al., 2016 ; Campos et al., 2017 ; Crippa et al., 2018 ; Fogaça et al., 2018 ; Sales et al., 2018 ). Interestingly, the rapid molecular changes induced by CBD are similar to several glutamatergic and GABAergic rapid-acting drugs discussed so far, whereas the long-term effects resemble monoaminergic drugs. For example, a single injection of CBD promotes synaptogenesis in the mPFC and induces rapid and sustained antidepressant effects through increased mTORC1/BDNF signaling ( Sales et al., 2018 ), and repeated administration of CBD prevents the decrease in neuronal remodeling/function and hippocampal neurogenesis induced by CUS ( Campos et al., 2013 ; Fogaça et al., 2018 ). In spite of these advances in the mechanism of action of CBD and other cannabinoid agents, there are very few studies that have investigated the role of the GABAergic system. Thus, more causal studies should be performed to determine the subtype of interneuron populations that mediate the anxiolytic and antidepressant effects of cannabinoid drugs, as well as other GABA-related cellular and synaptic mechanisms that could be involved.
Conclusion and Future Directions
For decades, the monoaminergic deficit hypothesis of depression was the prevalent theoretical basis for studies of the mechanisms underlying the pathophysiology and treatment of depression. However, although increased extracellular monoamines underlies the acute actions of monoamingergic agents, altered monoamine levels alone in forebrain areas are insufficient to explain the molecular and cellular changes underlying the antidepressant actions of these agents. Moreover, there is little consensus evidence that depression results from a deficit of monoamines. Thus, research has focused on neurotransmitter systems and microcircuits that can explain both the efficacy of antidepressant drugs and the etiology of MDD. Given growing consensus that MDD patients have a decrease in GABA levels in the brain and the revolutionary discovery that NMDA receptors antagonists, such as ketamine, can produce rapid and sustained antidepressant responses, efforts have been made to link the deficits in amino acid neurotransmitter systems to the pathophysiology of depression. Notably, the GABA deficit and the imbalance of cortical E:I hypothesis of depression provide a broader understanding of depression, as it offers connections with other important conceptual frameworks, such as altered glutamate and neurotrophic factor deficit hypotheses. With recent advances and new approaches, researchers have renewed enthusiasm for the development of fast-acting antidepressants that target the GABAergic and glutamatergic systems and overcome current therapeutic limitations of monoaminergic drugs. Despite recent advances, significant challenges remain, including development of more selective GABA, NMDA, and neuropeptide receptor agonists, antagonists, and modulators, characterization of optimal doses and treatment schedules, and better design of clinical trials. Moreover, genetic, chemogenetic, and optogenetic approaches should be directed to elucidate the role of specific interneuron subtypes and mechanisms underlying the control of behaviors related to mood and emotion, as well as sex-specific differences involved in these processes, with a view to developing more selective and improved antidepressant treatments.
Author Contributions
MF designed and wrote the manuscript, revised the literature, and prepared the figure and table. RD revised, edited, and approved the manuscript, figure, and table, and contributed in writing the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abdallah, C. G., Jackowski, A., Sato, J. R., Mao, X., Kang, G., Cheema, R., et al. (2015). Prefrontal cortical GABA abnormalities are associated with reduced hippocampal volume in major depressive disorder. Eur. Neuropsychopharmacol. 25, 1082–1090. doi: 10.1016/j.euroneuro.2015.04.025
PubMed Abstract | CrossRef Full Text | Google Scholar
Abellan, M. T., Adell, A., Honrubia, M. A., Mengod, G., and Artigas, F. (2000). GABAB-RI receptors in serotonergic neurons: effects of baclofen on 5-HT output in rat brain. Neuroreport 11, 941–945. doi: 10.1097/00001756-200004070-00009
Akana, S. F., Chu, A., Soriano, L., and Dallman, M. F. (2001). Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J. Neuroendocrinol. 13, 625–637. doi: 10.1046/j.1365-2826.2001.00676.x
Alexander, R. C. (2017). The potential efficacy of GABAB antagonists in depression. Curr. Opin. Pharmacol. 35, 101–104. doi: 10.1016/j.coph.2017.07.009
Almeida, F. B., Gomez, R., Barros, H. M. T., and Nin, M. S. (2019). Hemisphere-dependent changes in mRNA expression of GABAA receptor subunits and BDNF after intra-prefrontal cortex allopregnanolone infusion in rats. Neuroscience 397, 56–66. doi: 10.1016/j.neuroscience.2018.11.029
Arranz, B., Cowburn, R., Eriksson, A., Vestling, M., and Marcusson, J. (1992). Gamma-aminobutyric acid-B (GABAB) binding sites in postmortem suicide brains. Neuropsychobiology 26, 33–36. doi: 10.1159/000118893
Artigas, F., Bortolozzi, A., and Celada, P. (2018). Can we increase speed and efficacy of antidepressant treatments? Part I: general aspects and monoamine-based strategies. Eur. Neuropsychopharmacol. 28, 445–456. doi: 10.1016/j.euroneuro.2017.10.032
Atack, J. R. (2011). GABAA receptor subtype-selective modulators. I. alpha2/alpha3-selective agonists as non-sedating anxiolytics. Curr. Top. Med. Chem. 11, 1176–1202. doi: 10.2174/156802611795371350
Atack, J. R., Hallett, D. J., Tye, S., Wafford, K. A., Ryan, C., Sanabria-Bohorquez, S. M., et al. (2011). Preclinical and clinical pharmacology of TPA023B, a GABAA receptor α2/α3 subtype-selective partial agonist. J. Psychopharmacol. 25, 329–344. doi: 10.1177/0269881109354928
Atack, J. R., Maubach, K. A., Wafford, K. A., O’Connor, D., Rodrigues, A. D., Evans, D. C., et al. (2009). In vitro and in vivo properties of 3-tert-butyl-7-(5-methylisoxazol-3-yl)-2-(1-methyl-1H-1,2,4-triazol-5-ylmethoxy)- pyrazolo[1,5-d]-[1,2,4]triazine (MRK-016), a GABAA receptor alpha5 subtype-selective inverse agonist. J. Pharmacol. Exp. Ther. 331, 470–484. doi: 10.1124/jpet.109.157636
Autry, A. E., Adachi, M., Nosyreva, E., Na, E. S., Los, M. F., Cheng, P. F., et al. (2011). NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95. doi: 10.1038/nature10130
Bakas, T., van Nieuwenhuijzen, P. S., Devenish, S. O., McGregor, I. S., Arnold, J. C., and Chebib, M. (2017). The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol. Res. 119, 358–370. doi: 10.1016/j.phrs.2017.02.022
Banasr, M., Lepack, A., Fee, C., Duric, V., Maldonado-Aviles, J., DiLeone, R., et al. (2017). Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression. Chronic Stress 1. doi: 10.1177/2470547017720459
Banerjee, R., Ghosh, A. K., Ghosh, B., Bhattacharyya, S., and Mondal, A. C. (2013). Decreased mRNA and protein expression of BDNF, NGF, and their receptors in the hippocampus from suicide: an analysis in human postmortem brain. Clin. Med. Insights Pathol. 6, 1–11. doi: 10.4137/CMPath.S12530
Bauer, M., Bschor, T., Pfennig, A., Whybrow, P. C., Angst, J., Versiani, M., et al. (2007). World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders in primary care. World J. Biol. Psychiatry 8, 67–104. doi: 10.1080/15622970701227829
Beasley, C. L., Zhang, Z. J., Patten, I., and Reynolds, G. P. (2002). Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol. Psychiatry 52, 708–715. doi: 10.1016/S0006-3223(02)01360-4
CrossRef Full Text | Google Scholar
Benham, R. S., Hewage, N. B., Suckow, R. F., Engin, E., and Rudolph, U. (2017). Prodepressant- and anxiogenic-like effects of serotonin-selective, but not noradrenaline-selective, antidepressant agents in mice lacking alpha2-containing GABAA receptors. Behav. Brain Res. 332, 172–179. doi: 10.1016/j.bbr.2017.05.063
Berman, R. M., Cappiello, A., Anand, A., Oren, D. A., Heninger, G. R., Charney, D. S., et al. (2000). Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354. doi: 10.1016/S0006-3223(99)00230-9
Bhagwagar, Z., Wylezinska, M., Taylor, M., Jezzard, P., Matthews, P. M., and Cowen, P. J. (2004). Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am. J. Psychiatry 161, 368–370. doi: 10.1176/appi.ajp.161.2.368
Birkenhager, T. K., Moleman, P., and Nolen, W. A. (1995). Benzodiazepines for depression? A review of the literature. Int. Clin. Psychopharmacol. 10, 181–195. doi: 10.1097/00004850-199510030-00008
Bisogno, T., Hanus, L., De Petrocellis, L., Tchilibon, S., Ponde, D. E., Brandi, I., et al. (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 134, 845–852. doi: 10.1038/sj.bjp.0704327
Bittiger, H., Froestl, W., Mickel, S., and Olpe, H. R. (1993). GABAB receptor antagonists: from synthesis to therapeutic applications. Trends Pharmacol. Sci. 14, 391–394. doi: 10.1016/0165-6147(93)90056-P
Bowery, N. G., Doble, A., Hill, D. R., Hudson, A. L., Shaw, J. S., Turnbull, M. J., et al. (1981). Bicuculline-insensitive GABA receptors on peripheral autonomic nerve terminals. Eur. J. Pharmacol. 71, 53–70. doi: 10.1016/0014-2999(81)90386-1
Bowery, N. G., Hill, D. R., Hudson, A. L., Doble, A., Middlemiss, D. N., Shaw, J., et al. (1980). (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature 283, 92–94. doi: 10.1038/283092a0
Braestrup, C., Nielsen, M., Krogsgaard-Larsen, P., and Falch, E. (1979). Partial agonists for brain GABA/benzodiazepine receptor complex. Nature 280,331–333. doi: 10.1038/280331a0
Broqua, P., Wettstein, J. G., Rocher, M. N., Gauthier-Martin, B., and Junien, J. L. (1995). Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav. Pharmacol. 6, 215–222. doi: 10.1097/00008877-199504000-00001
Brown, J. A., Ramikie, T. S., Schmidt, M. J., Baldi, R., Garbett, K., Everheart, M. G., et al. (2015). Inhibition of parvalbumin-expressing interneurons results in complex behavioral changes. Mol. Psychiatry 20, 1499–1507. doi: 10.1038/mp.2014.192
Caberlotto, L., Jimenez, P., Overstreet, D. H., Hurd, Y. L., Mathe, A. A., and Fuxe, K. (1999). Alterations in neuropeptide Y levels and Y1 binding sites in the Flinders Sensitive Line rats, a genetic animal model of depression. Neurosci. Lett. 265, 191–194. doi: 10.1016/S0304-3940(99)00234-7
Caldji, C., Diorio, J., and Meaney, M. J. (2000). Variations in maternal care in infancy regulate the development of stress reactivity. Biol. Psychiatry 48, 1164–1174. doi: 10.1016/S0006-3223(00)01084-2
Caldji, C., Diorio, J., and Meaney, M. J. (2003). Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology 28, 1950–1959. doi: 10.1038/sj.npp.1300237
Campos, A. C., Fogaca, M. V., Scarante, F. F., Joca, S. R. L., Sales, A. J., Gomes, F. V., et al. (2017). Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front. Pharmacol. 8:269. doi: 10.3389/fphar.2017.00269
Campos, A. C., Ortega, Z., Palazuelos, J., Fogaca, M. V., Aguiar, D. C., Diaz-Alonso, J., et al. (2013). The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 16, 1407–1419. doi: 10.1017/S1461145712001502
Carlton, S. M., Zhou, S., Du, J., Hargett, G. L., Ji, G., and Coggeshall, R. E. (2004). Somatostatin modulates the transient receptor potential vanilloid 1 (TRPV1) ion channel. Pain 110, 616–627. doi: 10.1016/j.pain.2004.04.042
Celada, P., Puig, M. V., and Artigas, F. (2013). Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci. 7:25. doi: 10.3389/fnint.2013.00025
Celada, P., Puig, M. V., Casanovas, J. M., Guillazo, G., and Artigas, F. (2001). Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J. Neurosci. 21, 9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001
Chandra, D., Korpi, E. R., Miralles, C. P., De Blas, A. L., and Homanics, G. E. (2005). GABAA receptor gamma 2 subunit knockdown mice have enhanced anxiety-like behavior but unaltered hypnotic response to benzodiazepines. BMC Neurosci. 6:30. doi: 10.1186/1471-2202-6-30
Choudary, P. V., Molnar, M., Evans, S. J., Tomita, H., Li, J. Z., Vawter, M. P., et al. (2005). Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc. Natl. Acad. Sci. U.S.A. 102, 15653–15658. doi: 10.1073/pnas.0507901102
Cotter, D., Landau, S., Beasley, C., Stevenson, R., Chana, G., MacMillan, L., et al. (2002). The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol. Psychiatry 51, 377–386. doi: 10.1016/S0006-3223(01)01243-4
Crestani, F., Lorez, M., Baer, K., Essrich, C., Benke, D., Laurent, J. P., et al. (1999). Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 2, 833–839. doi: 10.1038/12207
Crippa, J. A., Guimaraes, F. S., Campos, A. C., and Zuardi, A. W. (2018). Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front. Immunol. 9:2009. doi: 10.3389/fimmu.2018.02009
Cross, J. A., Cheetham, S. C., Crompton, M. R., Katona, C. L., and Horton, R. W. (1988). Brain GABAB binding sites in depressed suicide victims. Psychiatry Res. 26, 119–129. doi: 10.1016/0165-1781(88)90066-2
Crowley, S. K., and Girdler, S. S. (2014). Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology 231, 3619–3634. doi: 10.1007/s00213-014-3572-8
Cryan, J. F., and Kaupmann, K. (2005). Don’t worry ’B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol. Sci. 26, 36–43. doi: 10.1016/j.tips.2004.11.004
Csabai, D., Seress, L., Varga, Z., Abraham, H., Miseta, A., Wiborg, O., et al. (2017). Electron microscopic analysis of hippocampal Axo-somatic synapses in a chronic stress model for depression. Hippocampus 27, 17–27. doi: 10.1002/hipo.22650
Czéh, B., Vardya, I., Varga, Z., Febbraro, F., Csabai, D., Martis, L. S., et al. (2018). Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front. Cell. Neurosci. 12:148. doi: 10.3389/fncel.2018.00148
Czéh, B., Varga, Z. K., Henningsen, K., Kovacs, G. L., Miseta, A., and Wiborg, O. (2015). Chronic stress reduces the number of GABAergic interneurons in the adult rat hippocampus, dorsal-ventral and region-specific differences. Hippocampus 25, 393–405. doi: 10.1002/hipo.22382
De Jong, M., Breeman, W. A., Bernard, H. F., Kooij, P. P., Slooter, G. D., Van Eijck, C. H., et al. (1999). Therapy of neuroendocrine tumors with radiolabeled somatostatin-analogues. Q. J. Nucl. Med. 43, 356–366.
Google Scholar
De Petrocellis, L., Ligresti, A., Moriello, A. S., Allara, M., Bisogno, T., Petrosino, S., et al. (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163, 1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x
DeFelipe, J., Lopez-Cruz, P. L., Benavides-Piccione, R., Bielza, C., Larranaga, P., Anderson, S., et al. (2013). New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 14, 202–216. doi: 10.1038/nrn3444
Diana, M. A., and Marty, A. (2004). Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br. J. Pharmacol. 142, 9–19. doi: 10.1038/sj.bjp.0705726
Diorio, D., Viau, V., and Meaney, M. J. (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 13, 3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993
Dong, E., Matsumoto, K., Uzunova, V., Sugaya, I., Takahata, H., Nomura, H., et al. (2001). Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc. Natl. Acad. Sci. U.S.A. 98, 2849–2854. doi: 10.1073/pnas.051628598
Douillard-Guilloux, G., Lewis, D., Seney, M. L., and Sibille, E. (2017). Decrease in somatostatin-positive cell density in the amygdala of females with major depression. Depress. Anxiety 34, 68–78. doi: 10.1002/da.22549
Drugan, R. C., Morrow, A. L., Weizman, R., Weizman, A., Deutsch, S. I., Crawley, J. N., et al. (1989). Stress-induced behavioral depression in the rat is associated with a decrease in GABA receptor-mediated chloride ion flux and brain benzodiazepine receptor occupancy. Brain Res. 487, 45–51. doi: 10.1016/0006-8993(89)90938-4
Dubin, M. J., Mao, X., Banerjee, S., Goodman, Z., Lapidus, K. A., Kang, G., et al. (2016). Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J. Psychiatry Neurosci. 41, E37–E45. doi: 10.1503/jpn.150223
Duman, R. S., Aghajanian, G. K., Sanacora, G., and Krystal, J. H. (2016). Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 22, 238–249. doi: 10.1038/nm.4050
Dwivedi, Y., Rizavi, H. S., Conley, R. R., Roberts, R. C., Tamminga, C. A., and Pandey, G. N. (2003). Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry 60, 804–815. doi: 10.1001/archpsyc.60.8.804
Engin, E., Benham, R. S., and Rudolph, U. (2018). An emerging circuit pharmacology of GABAA receptors. Trends Pharmacol. Sci. 39, 710–732. doi: 10.1016/j.tips.2018.04.003
Engin, E., Stellbrink, J., Treit, D., and Dickson, C. T. (2008). Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: behavioral and neurophysiological evidence. Neuroscience 157, 666–676. doi: 10.1016/j.neuroscience.2008.09.037
Engin, E., and Treit, D. (2009). Anxiolytic and antidepressant actions of somatostatin: the role of sst2 and sst3 receptors. Psychopharmacology 206, 281–289. doi: 10.1007/s00213-009-1605-5
Faron-Gorecka, A., Kusmider, M., Kolasa, M., Zurawek, D., Szafran-Pilch, K., Gruca, P., et al. (2016). Chronic mild stress alters the somatostatin receptors in the rat brain. Psychopharmacology 233, 255–266. doi: 10.1007/s00213-015-4103-y
Faron-Gorecka, A., Kusmider, M., Solich, J., Kolasa, M., Pabian, P., Gruca, P., et al. (2018). Regulation of somatostatin receptor 2 in the context of antidepressant treatment response in chronic mild stress in rat. Psychopharmacology 235, 2137–2149. doi: 10.1007/s00213-018-4912-x
Fatemi, S. H., Folsom, T. D., and Thuras, P. D. (2011). Deficits in GABA(B) receptor system in schizophrenia and mood disorders: a postmortem study. Schizophr. Res. 128, 37–43. doi: 10.1016/j.schres.2010.12.025
Fava, M., McCall, W. V., Krystal, A., Wessel, T., Rubens, R., Caron, J., et al. (2006). Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol. Psychiatry 59, 1052–1060. doi: 10.1016/j.biopsych.2006.01.016
Fava, M., Schaefer, K., Huang, H., Wilson, A., Iosifescu, D. V., Mischoulon, D., et al. (2011). A post hoc analysis of the effect of nightly administration of eszopiclone and a selective serotonin reuptake inhibitor in patients with insomnia and anxious depression. J. Clin. Psychiatry 72, 473–479. doi: 10.4088/JCP.09m05131gry
Fee, C., Banasr, M., and Sibille, E. (2017). Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biol. Psychiatry 82, 549–559. doi: 10.1016/j.biopsych.2017.05.024
Ferguson, B. R., and Gao, W. J. (2018). PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front. Neural Circuits 12:37. doi: 10.3389/fncir.2018.00037
Filipovic, D., Zlatkovic, J., Gass, P., and Inta, D. (2013). The differential effects of acute vs. chronic stress and their combination on hippocampal parvalbumin and inducible heat shock protein 70 expression. Neuroscience 236, 47–54. doi: 10.1016/j.neuroscience.2013.01.033
Fischell, J., Van Dyke, A. M., Kvarta, M. D., LeGates, T. A., and Thompson, S. M. (2015). Rapid antidepressant action and restoration of excitatory synaptic strength after chronic stress by negative modulators of alpha5-containing GABAA receptors. Neuropsychopharmacology 40, 2499–2509. doi: 10.1038/npp.2015.112
Fogaça, M. V., Campos, A. C., Coelho, L. D., Duman, R. S., and Guimaraes, F. S. (2018). The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: role of neurogenesis and dendritic remodeling. Neuropharmacology 135, 22–33. doi: 10.1016/j.neuropharm.2018.03.001
Fogaça, M. V., Lisboa, S. F., Aguiar, D. C., Moreira, F. A., Gomes, F. V., Casarotto, P. C., et al. (2012). Fine-tuning of defensive behaviors in the dorsal periaqueductal gray by atypical neurotransmitters. Braz. J. Med. Biol. Res. 45, 357–365. doi: 10.1590/S0100-879X2012007500029
Frankowska, M., Filip, M., and Przegalinski, E. (2007). Effects of GABAB receptor ligands in animal tests of depression and anxiety. Pharmacol. Rep. 59, 645–655.
PubMed Abstract | Google Scholar
Froestl, W., Gallagher, M., Jenkins, H., Madrid, A., Melcher, T., Teichman, S., et al. (2004). SGS742: the first GABA B receptor antagonist in clinical trials. Biochem. Pharmacol. 68, 1479–1487. doi: 10.1016/j.bcp.2004.07.030
Frye, M. A., Pazzaglia, P. J., George, M. S., Luckenbaugh, D. A., Vanderham, E., Davis, C. L., et al. (2003). Low CSF somatostatin associated with response to nimodipine in patents with affective illness. Biol. Psychiatry 53, 180–183. doi: 10.1016/S0006-3223(02)01343-4
Fuchs, T., Jefferson, S. J., Hooper, A., Yee, P. H., Maguire, J., and Luscher, B. (2017). Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol. Psychiatry 22, 920–930. doi: 10.1038/mp.2016.188
Fukumoto, K., Fogaca, M. V., Liu, R. J., Duman, C., Kato, T., Li, X. Y., et al. (2019). Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc. Natl. Acad. Sci. U.S.A. 116, 297–302. doi: 10.1073/pnas.1814709116
Gabbay, V., Mao, X., Klein, R. G., Ely, B. A., Babb, J. S., Panzer, A. M., et al. (2012). Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch. Gen. Psychiatry 69, 139–149. doi: 10.1001/archgenpsychiatry.2011.131
Gerner, R. H., and Hare, T. A. (1981). CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. Am. J. Psychiatry 138, 1098–1101. doi: 10.1176/ajp.138.8.1098
Ghosal, S., Bang, E., Yue, W., Hare, B. D., Lepack, A. E., Girgenti, M. J., et al. (2018). Activity-dependent brain-derived neurotrophic factor release is required for the rapid antidepressant actions of scopolamine. Biol. Psychiatry 83, 29–37. doi: 10.1016/j.biopsych.2017.06.017
Ghosal, S., Hare, B., and Duman, R. S. (2017). Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr. Opin. Behav. Sci. 14, 1–8. doi: 10.1016/j.cobeha.2016.09.012
Gilabert-Juan, J., Castillo-Gomez, E., Guirado, R., Molto, M. D., and Nacher, J. (2013). Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct. Funct. 218, 1591–1605. doi: 10.1007/s00429-012-0479-1
Gilabert-Juan, J., Varea, E., Guirado, R., Blasco-Ibanez, J. M., Crespo, C., and Nacher, J. (2012). Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neurosci. Lett. 530, 97–102. doi: 10.1016/j.neulet.2012.09.032
Godfrey, K. E. M., Gardner, A. C., Kwon, S., Chea, W., and Muthukumaraswamy, S. D. (2018). Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: a systematic review and meta-analysis. J. Psychiatr. Res. 105, 33–44. doi: 10.1016/j.jpsychires.2018.08.015
Gold, B. I., Bowers, M. B. Jr., Roth, R. H., and Sweeney, D. W. (1980). GABA levels in CSF of patients with psychiatric disorders. Am. J. Psychiatry 137, 362–364. doi: 10.1176/ajp.137.3.362
Goren, M. Z., Kucukibrahimoglu, E., Berkman, K., and Terzioglu, B. (2007). Fluoxetine partly exerts its actions through GABA: a neurochemical evidence. Neurochem. Res. 32, 1559–1565. doi: 10.1007/s11064-007-9357-2
Gray, J. A., and Green, A. R. (1987). Increased GABAB receptor function in mouse frontal cortex after repeated administration of antidepressant drugs or electroconvulsive shocks. Br. J. Pharmacol. 92, 357–362. doi: 10.1111/j.1476-5381.1987.tb11331.x
Griffin, L. D., and Mellon, S. H. (1999). Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc. Natl. Acad. Sci. U.S.A. 96, 13512–13517. doi: 10.1073/pnas.96.23.13512
Guidotti, A., Dong, E., Matsumoto, K., Pinna, G., Rasmusson, A. M., and Costa, E. (2001). The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5alpha-dihydroprogesterone in psychiatric disorders. Brain Res. Brain Res. Rev. 37, 110–115. doi: 10.1016/S0165-0173(01)00129-1
Guilloux, J. P., Douillard-Guilloux, G., Kota, R., Wang, X., Gardier, A. M., Martinowich, K., et al. (2012). Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatry 17, 1130–1142. doi: 10.1038/mp.2011.113
Hallschmid, M., Benedict, C., Born, J., Fehm, H. L., and Kern, W. (2004). Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol. Behav. 83, 55–64. doi: 10.1016/S0031-9384(04)00349-X
Hallschmid, M., Gais, S., Meinert, S., and Born, J. (2003). NPY attenuates positive cortical DC-potential shift upon food intake in man. Psychoneuroendocrinology 28, 529–539. doi: 10.1016/S0306-4530(02)00038-0
Harmer, C. J., Duman, R. S., and Cowen, P. J. (2017). How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 4, 409–418. doi: 10.1016/S2215-0366(17)30015-9
Hasler, G., van der Veen, J. W., Grillon, C., Drevets, W. C., and Shen, J. (2010). Effect of acute psychological stress on prefrontal GABA concentration determined by proton magnetic resonance spectroscopy. Am. J. Psychiatry 167, 1226–1231. doi: 10.1176/appi.ajp.2010.09070994
Hasler, G., van der Veen, J. W., Tumonis, T., Meyers, N., Shen, J., and Drevets, W. C. (2007). Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 64, 193–200. doi: 10.1001/archpsyc.64.2.193
Heese, K., Otten, U., Mathivet, P., Raiteri, M., Marescaux, C., and Bernasconi, R. (2000). GABA(B) receptor antagonists elevate both mRNA and protein levels of the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) but not neurotrophin-3 (NT-3) in brain and spinal cord of rats. Neuropharmacology 39, 449–462. doi: 10.1016/S0028-3908(99)00166-5
Heilig, M., McLeod, S., Koob, G. K., and Britton, K. T. (1992). Anxiolytic-like effect of neuropeptide Y (NPY), but not other peptides in an operant conflict test. Regul. Pept. 41, 61–69. doi: 10.1016/0167-0115(92)90514-U
Heilig, M., and Murison, R. (1987). Intracerebroventricular neuropeptide Y protects against stress-induced gastric erosion in the rat. Eur. J. Pharmacol. 137, 127–129. doi: 10.1016/0014-2999(87)90191-9
Heilig, M., Soderpalm, B., Engel, J. A., and Widerlov, E. (1989). Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology 98, 524–529. doi: 10.1007/BF00441953
Heilig, M., Zachrisson, O., Thorsell, A., Ehnvall, A., Mottagui-Tabar, S., Sjögren, M., et al. (2004). Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J. Psychiatr. Res. 38, 113–121. doi: 10.1016/S0022-3956(03)00101-8
Heinzel, A., Steinke, R., Poeppel, T. D., Grosser, O., Bogerts, B., Otto, H., et al. (2008). S-ketamine and GABA-A-receptor interaction in humans: an exploratory study with I-123-iomazenil SPECT. Hum. Psychopharmacol. 23, 549–554. doi: 10.1002/hup.960
Hewitt, S. A., Wamsteeker, J. I., Kurz, E. U., and Bains, J. S. (2009). Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat. Neurosci. 12, 438–443. doi: 10.1038/nn.2274
Hill, E. L., Gallopin, T., Ferezou, I., Cauli, B., Rossier, J., Schweitzer, P., et al. (2007). Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J. Neurophysiol. 97, 2580–2589. doi: 10.1152/jn.00603.2006
Hill, M. N., McLaughlin, R. J., Pan, B., Fitzgerald, M. L., Roberts, C. J., Lee, T. T., et al. (2011). Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 31, 10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011
Hofland, L. J., van Koetsveld, P. M., Wouters, N., Waaijers, M., Reubi, J. C., and Lamberts, S. W. (1992). Dissociation of antiproliferative and antihormonal effects of the somatostatin analog octreotide on 7315b pituitary tumor cells. Endocrinology 131, 571–577.
Jacobson, L. H., Vlachou, S., Slattery, D. A., Li, X., and Cryan, J. F. (2018). The gamma-aminobutyric acid b receptor in depression and reward. Biol. Psychiatry 83, 963–976. doi: 10.1016/j.biopsych.2018.02.006
Jiang, N., Furue, H., Katafuchi, T., and Yoshimura, M. (2003). Somatostatin directly inhibits substantia gelatinosa neurons in adult rat spinal dorsal horn in vitro. Neurosci. Res. 47, 97–107. doi: 10.1016/S0168-0102(03)00183-4
Jonas, J. M., and Cohon, M. S. (1993). A comparison of the safety and efficacy of alprazolam versus other agents in the treatment of anxiety, panic, and depression: a review of the literature. J. Clin. Psychiatry 54(Suppl.), 25–45; discussion 46–28.
Jovanovic, J. N., Thomas, P., Kittler, J. T., Smart, T. G., and Moss, S. J. (2004). Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J. Neurosci. 24, 522–530. doi: 10.1523/JNEUROSCI.3606-03.2004
Kanes, S., Colquhoun, H., Gunduz-Bruce, H., Raines, S., Arnold, R., Schacterle, A., et al. (2017). Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet 390, 480–489. doi: 10.1016/S0140-6736(17)31264-3
Kanes, S. J., Colquhoun, H., Doherty, J., Raines, S., Hoffmann, E., Rubinow, D. R., et al. (2017). Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Hum. Psychopharmacol. 32:e2576. doi: 10.1002/hup.2576
Karch, D. L., Dahlberg, L. L., Patel, N., Davis, T. W., Logan, J. E., Hill, H. A., et al. (2009). Surveillance for violent deaths–national violent death reporting system, 16 States, 2006. MMWR Surveill. Summ. 58, 1–44.
Karlsson, R. M., Choe, J. S., Cameron, H. A., Thorsell, A., Crawley, J. N., Holmes, A., et al. (2008). The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology 195, 547–557. doi: 10.1007/s00213-007-0945-2
Karolewicz, B., Maciag, D., O’Dwyer, G., Stockmeier, C. A., Feyissa, A. M., and Rajkowska, G. (2010). Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int. J. Neuropsychopharmacol. 13, 411–420. doi: 10.1017/S1461145709990587
Kato, T., Fogaca, M. V., Deyama, S., Li, X. Y., Fukumoto, K., and Duman, R. S. (2018). BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol. Psychiatry 23, 2007–2017. doi: 10.1038/mp.2017.220
Kaupmann, K., Huggel, K., Heid, J., Flor, P. J., Bischoff, S., Mickel, S. J., et al. (1997). Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature 386, 239–246. doi: 10.1038/386239a0
Kessler, R. C., Chiu, W. T., Demler, O., Merikangas, K. R., and Walters, E. E. (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 617–627. doi: 10.1001/archpsyc.62.6.617
Khisti, R. T., Chopde, C. T., and Jain, S. P. (2000). Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol. Biochem. Behav. 67, 137–143. doi: 10.1016/S0091-3057(00)00300-2
Kim, Y. K., Lee, H. P., Won, S. D., Park, E. Y., Lee, H. Y., Lee, B. H., et al. (2007). Low plasma BDNF is associated with suicidal behavior in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 78–85.
Klempan, T. A., Sequeira, A., Canetti, L., Lalovic, A., Ernst, C., ffrench-Mullen, J., et al. (2009). Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol. Psychiatry 14, 175–189. doi: 10.1038/sj.mp.4002110
Krystal, A., Fava, M., Rubens, R., Wessel, T., Caron, J., Wilson, P., et al. (2007). Evaluation of eszopiclone discontinuation after cotherapy with fluoxetine for insomnia with coexisting depression. J. Clin. Sleep Med. 3, 48–55.
Kucukibrahimoglu, E., Saygin, M. Z., Caliskan, M., Kaplan, O. K., Unsal, C., and Goren, M. Z. (2009). The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur. J. Clin. Pharmacol. 65, 571–577. doi: 10.1007/s00228-009-0650-7
Kuehner, C. (2017). Why is depression more common among women than among men? Lancet Psychiatry 4, 146–158. doi: 10.1016/S2215-0366(16)30263-2
Lacroix, J. S., and Mosimann, B. L. (1996). Attenuation of allergen-evoked nasal responses by local pretreatment with exogenous neuropeptide Y in atopic patients. J. Allergy Clin. Immunol. 98, 611–616. doi: 10.1016/S0091-6749(96)70095-7
Lacroix, J. S., Ricchetti, A. P., Morel, D., Mossimann, B., Waeber, B., and Grouzmann, E. (1996). Intranasal administration of neuropeptide Y in man: systemic absorption and functional effects. Br. J. Pharmacol. 118, 2079–2084. doi: 10.1111/j.1476-5381.1996.tb15647.x
Larhammar, D., and Salaneck, E. (2004). Molecular evolution of NPY receptor subtypes. Neuropeptides 38, 141–151. doi: 10.1016/j.npep.2004.06.002
Lepack, A. E., Bang, E., Lee, B., Dwyer, J. M., and Duman, R. S. (2016). Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology 111, 242–252. doi: 10.1016/j.neuropharm.2016.09.011
Lepack, A. E., Fuchikami, M., Dwyer, J. M., Banasr, M., and Duman, R. S. (2014). BDNF release is required for the behavioral actions of ketamine. Int. J. Neuropsychopharmacol. 18:yu033. doi: 10.1093/ijnp/pyu033
Li, N., Lee, B., Liu, R. J., Banasr, M., Dwyer, J. M., Iwata, M., et al. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964. doi: 10.1126/science.1190287
Lin, L. C., and Sibille, E. (2015). Somatostatin, neuronal vulnerability and behavioral emotionality. Mol. Psychiatry 20, 377–387. doi: 10.1038/mp.2014.184
Liu, C. Y., Jiang, X. X., Zhu, Y. H., and Wei, D. N. (2012). Metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine produces antidepressant effects in rats: role of brain-derived neurotrophic factor. Neuroscience 223, 219–224. doi: 10.1016/j.neuroscience.2012.08.010
Llado-Pelfort, L., Santana, N., Ghisi, V., Artigas, F., and Celada, P. (2012). 5-HT1A receptor agonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb. Cortex 22, 1487–1497. doi: 10.1093/cercor/bhr220
Lloyd, K. G., Thuret, F., and Pilc, A. (1985). Upregulation of gamma-aminobutyric acid (GABA) B binding sites in rat frontal cortex: a common action of repeated administration of different classes of antidepressants and electroshock. J. Pharmacol. Exp. Ther. 235, 191–199.
Luscher, B., Shen, Q., and Sahir, N. (2011). The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 16, 383–406. doi: 10.1038/mp.2010.120
Ma, K., Xu, A., Cui, S., Sun, M. R., Xue, Y. C., and Wang, J. H. (2016). Impaired GABA synthesis, uptake and release are associated with depression-like behaviors induced by chronic mild stress. Transl. Psychiatry 6:e910. doi: 10.1038/tp.2016.181
Maciag, D., Hughes, J., O’Dwyer, G., Pride, Y., Stockmeier, C. A., Sanacora, G., et al. (2010). Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Biol. Psychiatry 67, 465–470. doi: 10.1016/j.biopsych.2009.10.027
MacQueen, G. M., Yucel, K., Taylor, V. H., Macdonald, K., and Joffe, R. (2008). Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol. Psychiatry 64, 880–883. doi: 10.1016/j.biopsych.2008.06.027
Maggi, A., and Perez, J. (1986). Estrogen-induced up-regulation of gamma-aminobutyric acid receptors in the CNS of rodents. J. Neurochem. 47,1793–1797. doi: 10.1111/j.1471-4159.1986.tb13090.x
Maguire, J., and Mody, I. (2007). Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J. Neurosci. 27, 2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007
Markram, H., Toledo-Rodriguez, M., Wang, Y., Gupta, A., Silberberg, G., and Wu, C. (2004). Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807. doi: 10.1038/nrn1519
Martin, P., Pichat, P., Massol, J., Soubrie, P., Lloyd, K. G., and Puech, A. J. (1989). Decreased GABA B receptors in helpless rats: reversal by tricyclic antidepressants. Neuropsychobiology 22, 220–224. doi: 10.1159/000118620
Matsumoto, K., Puia, G., Dong, E., and Pinna, G. (2007). GABA(A) receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress 10, 3–12. doi: 10.1080/10253890701200997
McKlveen, J. M., Morano, R. L., Fitzgerald, M., Zoubovsky, S., Cassella, S. N., Scheimann, J. R., et al. (2016). Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol. Psychiatry 80, 754–764. doi: 10.1016/j.biopsych.2016.03.2101
Meis, S., Sosulina, L., Schulz, S., Hollt, V., and Pape, H. C. (2005). Mechanisms of somatostatin-evoked responses in neurons of the rat lateral amygdala. Eur. J. Neurosci. 21, 755–762. doi: 10.1111/j.1460-9568.2005.03922.x
Melas, P. A., Mannervik, M., Mathe, A. A., and Lavebratt, C. (2012). Neuropeptide Y: identification of a novel rat mRNA splice-variant that is downregulated in the hippocampus and the prefrontal cortex of a depression-like model. Peptides 35, 49–55. doi: 10.1016/j.peptides.2012.02.020
Meltzer-Brody, S., Colquhoun, H., Riesenberg, R., Epperson, C. N., Deligiannidis, K. M., Rubinow, D. R., et al. (2018). Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 392, 1058–1070. doi: 10.1016/S0140-6736(18)31551-4
Merali, Z., Du, L., Hrdina, P., Palkovits, M., Faludi, G., Poulter, M. O., et al. (2004). Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J. Neurosci. 24, 1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004
Milak, M. S., Proper, C. J., Mulhern, S. T., Parter, A. L., Kegeles, L. S., Ogden, R. T., et al. (2016). A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol. Psychiatry 21, 320–327. doi: 10.1038/mp.2015.83
Moghaddam, B., Adams, B., Verma, A., and Daly, D. (1997). Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 17, 2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997
Mohler, H., Fritschy, J. M., and Rudolph, U. (2002). A new benzodiazepine pharmacology. J. Pharmacol. Exp. Ther. 300, 2–8. doi: 10.1124/jpet.300.1.2
Mombereau, C., Kaupmann, K., Froestl, W., Sansig, G., van der Putten, H., and Cryan, J. F. (2004). Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology 29, 1050–1062. doi: 10.1038/sj.npp.1300413
Naert, G., Maurice, T., Tapia-Arancibia, L., and Givalois, L. (2007). Neuroactive steroids modulate HPA axis activity and cerebral brain-derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology 32, 1062–1078. doi: 10.1016/j.psyneuen.2007.09.002
Nakagawa, Y., Sasaki, A., and Takashima, T. (1999). The GABA(B) receptor antagonist CGP36742 improves learned helplessness in rats. Eur. J. Pharmacol. 381, 1–7. doi: 10.1016/S0014-2999(99)00567-1
Nikisch, G., Agren, H., Eap, C. B., Czernik, A., Baumann, P., and Mathe, A. A. (2005). Neuropeptide Y and corticotropin-releasing hormone in CSF mark response to antidepressive treatment with citalopram. Int. J. Neuropsychopharmacol. 8, 403–410. doi: 10.1017/S1461145705005158
Nin, M. S., Martinez, L. A., Pibiri, F., Nelson, M., and Pinna, G. (2011). Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front. Endocrinol. 2:73. doi: 10.3389/fendo.2011.00073
Northoff, G., and Sibille, E. (2014). Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol. Psychiatry 19, 966–977. doi: 10.1038/mp.2014.68
Nowak, B., Zadrozna, M., Ossowska, G., Sowa-Kucma, M., Gruca, P., Papp, M., et al. (2010). Alterations in hippocampal calcium-binding neurons induced by stress models of depression: a preliminary assessment. Pharmacol. Rep. 62, 1204–1210. doi: 10.1016/S1734-1140(10)70383-2
Nowak, G., Partyka, A., Palucha, A., Szewczyk, B., Wieronska, J. M., Dybala, M., et al. (2006). Antidepressant-like activity of CGP 36742 and CGP 51176, selective GABAB receptor antagonists, in rodents. Br. J. Pharmacol. 149,581–590. doi: 10.1038/sj.bjp.0706845
Nyitrai, G., Kekesi, K. A., Emri, Z., Szarics, E., Juhasz, G., and Kardos, J. (2003). GABA(B) receptor antagonist CGP-36742 enhances somatostatin release in the rat hippocampus in vivo and in vitro. Eur. J. Pharmacol. 478, 111–119. doi: 10.1016/j.ejphar.2003.08.006
Nyitrai, G., Szarics, E., Kovacs, I., Kekesi, K. A., Juhasz, G., and Kardos, J. (1999). Effect of CGP 36742 on the extracellular level of neurotransmitter amino acids in the thalamus. Neurochem. Int. 34, 391–398. doi: 10.1016/S0197-0186(99)00042-X
Ordway, G. A., Stockmeier, C. A., Meltzer, H. Y., Overholser, J. C., Jaconetta, S., and Widdowson, P. S. (1995). Neuropeptide Y in frontal cortex is not altered in major depression. J. Neurochem. 65, 1646–1650. doi: 10.1046/j.1471-4159.1995.65041646.x
Otero Losada, M. E. (1988). Changes in central GABAergic function following acute and repeated stress. Br. J. Pharmacol. 93, 483–490. doi: 10.1111/j.1476-5381.1988.tb10302.x
Pawlikowski, M., and Melen-Mucha, G. (2003). Perspectives of new potential therapeutic applications of somatostatin analogs. Neuro Endocrinol. Lett. 24, 21–27.
Pehrson, A. L., and Sanchez, C. (2015). Altered gamma-aminobutyric acid neurotransmission in major depressive disorder: a critical review of the supporting evidence and the influence of serotonergic antidepressants. Drug Des. Devel. Ther. 9, 603–624. doi: 10.2147/DDDT.S62912
Perova, Z., Delevich, K., and Li, B. (2015). Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. J. Neurosci. 35, 3201–3206. doi: 10.1523/JNEUROSCI.2670-14.2015
Perrine, S. A., Ghoddoussi, F., Michaels, M. S., Sheikh, I. S., McKelvey, G., and Galloway, M. P. (2014). Ketamine reverses stress-induced depression-like behavior and increased GABA levels in the anterior cingulate: an 11.7 T 1H-MRS study in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 51, 9–15. doi: 10.1016/j.pnpbp.2013.11.003
Pettit, D. A., Harrison, M. P., Olson, J. M., Spencer, R. F., and Cabral, G. A. (1998). Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J. Neurosci. Res. 51, 391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A
Petty, F., and Sherman, A. D. (1984). Plasma GABA levels in psychiatric illness. J. Affect. Disord. 6, 131–138. doi: 10.1016/0165-0327(84)90018-1
Petty, F., Trivedi, M. H., Fulton, M., and Rush, A. J. (1995). Benzodiazepines as antidepressants: does GABA play a role in depression? Biol. Psychiatry 38, 578–591.
Piantadosi, S. C., French, B. J., Poe, M. M., Timic, T., Markovic, B. D., Pabba, M., et al. (2016). Sex-dependent anti-stress effect of an alpha5 subunit containing GABAA receptor positive allosteric modulator. Front. Pharmacol. 7:446. doi: 10.3389/fphar.2016.00446
Pich, E. M., Agnati, L. F., Zini, I., Marrama, P., and Carani, C. (1993). Neuropeptide Y produces anxiolytic effects in spontaneously hypertensive rats. Peptides 14, 909–912. doi: 10.1016/0196-9781(93)90065-O
Pinna, G., Costa, E., and Guidotti, A. (2006). Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology 186, 362–372. doi: 10.1007/s00213-005-0213-2
Pinna, G., Costa, E., and Guidotti, A. (2009). SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr. Opin. Pharmacol. 9, 24–30. doi: 10.1016/j.coph.2008.12.006
Pinter, E., Helyes, Z., and Szolcsanyi, J. (2006). Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol. Ther. 112, 440–456. doi: 10.1016/j.pharmthera.2006.04.010
Poleszak, E., Wosko, S., Slawinska, K., Szopa, A., Wrobel, A., and Serefko, A. (2018). Cannabinoids in depressive disorders. Life Sci. 213, 18–24. doi: 10.1016/j.lfs.2018.09.058
Post, R. M., Ketter, T. A., Joffe, R. T., and Kramlinger, K. L. (1991). Lack of beneficial effects of l-baclofen in affective disorder. Int. Clin. Psychopharmacol. 6, 197–207. doi: 10.1097/00004850-199100640-00001
Prescot, A., Sheth, C., Legarreta, M., Renshaw, P. F., McGlade, E., and Yurgelun-Todd, D. (2018). Altered cortical gamma-amino butyric acid in female veterans with suicidal behavior: sex differences and clinical correlates. Chronic Stress 2. doi: 10.1177/2470547018768771
Prevot, T. D., Gastambide, F., Viollet, C., Henkous, N., Martel, G., Epelbaum, J., et al. (2017). Roles of hippocampal somatostatin receptor subtypes in stress response and emotionality. Neuropsychopharmacology 42, 1647–1656. doi: 10.1038/npp.2016.281
Puig, M. V., Santana, N., Celada, P., Mengod, G., and Artigas, F. (2004). In vivo excitation of GABA interneurons in the medial prefrontal cortex through 5-HT3 receptors. Cereb. Cortex 14, 1365–1375. doi: 10.1093/cercor/bhh097
Radley, J. J., Gosselink, K. L., and Sawchenko, P. E. (2009). A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J. Neurosci. 29, 7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009
Rajkowska, G., O’Dwyer, G., Teleki, Z., Stockmeier, C. A., and Miguel-Hidalgo, J. J. (2007). GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 32, 471–482. doi: 10.1038/sj.npp.1301234
Reddy, M. S. (2010). Depression: the disorder and the burden. Indian J. Psychol. Med. 32, 1–2. doi: 10.4103/0253-7176.70510
Redrobe, J. P., Dumont, Y., Fournier, A., and Quirion, R. (2002a). The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology 26, 615–624.
Redrobe, J. P., Dumont, Y., and Quirion, R. (2002b). Neuropeptide Y (NPY) and depression: from animal studies to the human condition. Life Sci. 71, 2921–2937.
Ren, Z., Pribiag, H., Jefferson, S. J., Shorey, M., Fuchs, T., Stellwagen, D., et al. (2016). Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol. Psychiatry 80, 457–468. doi: 10.1016/j.biopsych.2016.02.009
Robinson, R. T., Drafts, B. C., and Fisher, J. L. (2003). Fluoxetine increases GABA(A) receptor activity through a novel modulatory site. J. Pharmacol. Exp. Ther. 304, 978–984. doi: 10.1124/jpet.102.044834
Rodriguez-Landa, J. F., Contreras, C. M., Bernal-Morales, B., Gutierrez-Garcia, A. G., and Saavedra, M. (2007). Allopregnanolone reduces immobility in the forced swimming test and increases the firing rate of lateral septal neurons through actions on the GABAA receptor in the rat. J. Psychopharmacol. 21, 76–84. doi: 10.1177/0269881106064203
Romeo, E., Strohle, A., Spalletta, G., di Michele, F., Hermann, B., Holsboer, F., et al. (1998). Effects of antidepressant treatment on neuroactive steroids in major depression. Am. J. Psychiatry 155, 910–913. doi: 10.1176/ajp.155.7.910
Rosa, P. B., Neis, V. B., Ribeiro, C. M., Moretti, M., and Rodrigues, A. L. (2016). Antidepressant-like effects of ascorbic acid and ketamine involve modulation of GABAA and GABAB receptors. Pharmacol. Rep. 68, 996–1001. doi: 10.1016/j.pharep.2016.05.010
Rubinow, D. R., Gold, P. W., Post, R. M., and Ballenger, J. C. (1985). CSF somatostatin in affective illness and normal volunteers. Prog. Neuropsychopharmacol. Biol. Psychiatry 9, 393–400.
Rudolph, U., and Knoflach, F. (2011). Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 10, 685–697. doi: 10.1038/nrd3502
Rudy, B., Fishell, G., Lee, S., and Hjerling-Leffler, J. (2011). Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 71, 45–61. doi: 10.1002/dneu.20853
Russo, E. B., Burnett, A., Hall, B., and Parker, K. K. (2005). Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 30, 1037–1043. doi: 10.1007/s11064-005-6978-1
Sage Therapeutics (2017). Sage Therapeutics Reports Positive Top-Line Results from Phase 2 Placebo-Controlled Trial of SAGE-217 in Major Depressive Disorder [Press Release]. Cambridge, MA: Sage Therapeutics.
Sajdyk, T. J., Johnson, P. L., Leitermann, R. J., Fitz, S. D., Dietrich, A., Morin, M., et al. (2008). Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J. Neurosci. 28, 893–903. doi: 10.1523/JNEUROSCI.0659-07.2008
Sales, A. J., Fogaca, M. V., Sartim, A. G., Pereira, V. S., Wegener, G., Guimaraes, F. S., et al. (2018). Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol. Neurobiol. doi: 10.1007/s12035-018-1143-4 [Epub ahead of print].
Sanacora, G., Gueorguieva, R., Epperson, C. N., Wu, Y. T., Appel, M., Rothman, D. L., et al. (2004). Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry 61, 705–713. doi: 10.1001/archpsyc.61.7.705
Sanacora, G., Mason, G. F., Rothman, D. L., Behar, K. L., Hyder, F., Petroff, O. A., et al. (1999). Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 56, 1043–1047. doi: 10.1001/archpsyc.56.11.1043
Santana, N., Bortolozzi, A., Serrats, J., Mengod, G., and Artigas, F. (2004). Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex 14, 1100–1109. doi: 10.1093/cercor/bhh070
Savitz, J., and Drevets, W. C. (2009). Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci. Biobehav. Rev. 33, 699–771. doi: 10.1016/j.neubiorev.2009.01.004
Sayed, S., Van Dam, N. T., Horn, S. R., Kautz, M. M., Parides, M., Costi, S., et al. (2018). A randomized dose-ranging study of neuropeptide Y in patients with posttraumatic stress disorder. Int. J. Neuropsychopharmacol. 21, 3–11. doi: 10.1093/ijnp/pyx109
Schiavon, A. P., Bonato, J. M., Milani, H., Guimaraes, F. S., and Weffort de Oliveira, R. M. (2016). Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 27–34. doi: 10.1016/j.pnpbp.2015.06.017
Sequeira, A., Klempan, T., Canetti, L., ffrench-Mullen, J., Benkelfat, C., Rouleau, G. A., et al. (2007). Patterns of gene expression in the limbic system of suicides with and without major depression. Mol. Psychiatry 12, 640–655. doi: 10.1038/sj.mp.4001969
Serrats, J., Artigas, F., Mengod, G., and Cortes, R. (2003). GABAB receptor mRNA in the raphe nuclei: co-expression with serotonin transporter and glutamic acid decarboxylase. J. Neurochem. 84, 743–752. doi: 10.1046/j.1471-4159.2003.01557.x
Shalaby, A., and Kamal, S. (2009). Effect of Escitalopram on GABA level and anti-oxidant markers in prefrontal cortex and nucleus accumbens of chronic mild stress-exposed albino rats. Int. J. Physiol. Pathophysiol. Pharmacol. 1, 154–161.
Shen, Q., Lal, R., Luellen, B. A., Earnheart, J. C., Andrews, A. M., and Luscher, B. (2010). gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol. Psychiatry 68, 512–520. doi: 10.1016/j.biopsych.2010.04.024
Shepard, R., and Coutellier, L. (2018). Changes in the prefrontal glutamatergic and parvalbumin systems of mice exposed to unpredictable chronic stress. Mol. Neurobiol. 55, 2591–2602. doi: 10.1007/s12035-017-0528-0
Shepard, R., Page, C. E., and Coutellier, L. (2016). Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: relevance for sex differences in stress-related disorders. Neuroscience 332, 1–12. doi: 10.1016/j.neuroscience.2016.06.038
Shimizu, E., Hashimoto, K., Okamura, N., Koike, K., Komatsu, N., Kumakiri, C., et al. (2003). Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry 54, 70–75. doi: 10.1016/S0006-3223(03)00181-1
Sibille, E., Morris, H. M., Kota, R. S., and Lewis, D. A. (2011). GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int. J. Neuropsychopharmacol. 14, 721–734. doi: 10.1017/S1461145710001616
Slattery, D. A., Desrayaud, S., and Cryan, J. F. (2005). GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. J. Pharmacol. Exp. Ther. 312, 290–296. doi: 10.1124/jpet.104.073536
Smith, K. S., and Rudolph, U. (2012). Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology 62, 54–62. doi: 10.1016/j.neuropharm.2011.07.026
Song, Z., Huang, P., Qiu, L., Wu, Q., Gong, Q., Zhang, B., et al. (2012). [Decreased occipital GABA concentrations in patients with first-episode major depressive disorder: a magnetic resonance spectroscopy study]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 29, 233–236.
Soumier, A., and Sibille, E. (2014). Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology 39, 2252–2262. doi: 10.1038/npp.2014.76
Stampanoni Bassi, M., Gilio, L., Maffei, P., Dolcetti, E., Bruno, A., Buttari, F., et al. (2018). Exploiting the multifaceted effects of cannabinoids on mood to boost their therapeutic use against anxiety and depression. Front. Mol. Neurosci. 11:424. doi: 10.3389/fnmol.2018.00424
Stone, J. M., Dietrich, C., Edden, R., Mehta, M. A., De Simoni, S., Reed, L. J., et al. (2012). Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol. Psychiatry 17, 664–665. doi: 10.1038/mp.2011.171
Strohle, A., Romeo, E., Hermann, B., Pasini, A., Spalletta, G., di Michele, F., et al. (1999). Concentrations of 3 alpha-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol. Psychiatry 45, 274–277. doi: 10.1016/S0006-3223(98)00328-X
Szabo, B., and Schlicker, E. (2005). Effects of cannabinoids on neurotransmission. Handb. Exp. Pharmacol. 168, 327–365. doi: 10.1007/3-540-26573-2_11
Taylor, M. J., Tiangga, E. R., Mhuircheartaigh, R. N., and Cowen, P. J. (2012). Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J. Psychopharmacol. 26, 733–737. doi: 10.1177/0269881111405359
Thase, M. E., Mahableshwarkar, A. R., Dragheim, M., Loft, H., and Vieta, E. (2016). A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur. Neuropsychopharmacol. 26, 979–993. doi: 10.1016/j.euroneuro.2016.03.007
Thorsell, A., Michalkiewicz, M., Dumont, Y., Quirion, R., Caberlotto, L., Rimondini, R., et al. (2000). Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc. Natl. Acad. Sci. U.S.A. 97, 12852–12857. doi: 10.1073/pnas.220232997
Todorovic, N., Micic, B., Schwirtlich, M., Stevanovic, M., and Filipovic, D. (2019). Subregion-specific protective effects of fluoxetine and clozapine on parvalbumin expression in medial prefrontal cortex of chronically isolated rats. Neuroscience 396, 24–35. doi: 10.1016/j.neuroscience.2018.11.008
Tremblay, R., Lee, S., and Rudy, B. (2016). GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91, 260–292. doi: 10.1016/j.neuron.2016.06.033
Tripp, A., Kota, R. S., Lewis, D. A., and Sibille, E. (2011). Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol. Dis. 42, 116–124. doi: 10.1016/j.nbd.2011.01.014
Tripp, A., Oh, H., Guilloux, J. P., Martinowich, K., Lewis, D. A., and Sibille, E. (2012). Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am. J. Psychiatry 169, 1194–1202. doi: 10.1176/appi.ajp.2012.12020248
Trivedi, M. H., Rush, A. J., Wisniewski, S. R., Nierenberg, A. A., Warden, D., Ritz, L., et al. (2006). Evaluation of outcomes with citalopram for depression using measurement-based care in STAR ∗ D: implications for clinical practice. Am. J. Psychiatry 163, 28–40. doi: 10.1176/appi.ajp.163.1.28
Urban-Ciecko, J., and Barth, A. L. (2016). Somatostatin-expressing neurons in cortical networks. Nat. Rev. Neurosci. 17, 401–409. doi: 10.1038/nrn.2016.53
Uzunov, D. P., Cooper, T. B., Costa, E., and Guidotti, A. (1996). Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc. Natl. Acad. Sci. U.S.A. 93, 12599–12604. doi: 10.1073/pnas.93.22.12599
Uzunova, V., Sheline, Y., Davis, J. M., Rasmusson, A., Uzunov, D. P., Costa, E., et al. (1998). Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl. Acad. Sci. U.S.A. 95, 3239–3244. doi: 10.1073/pnas.95.6.3239
Uzunova, V., Wrynn, A. S., Kinnunen, A., Ceci, M., Kohler, C., and Uzunov, D. P. (2004). Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur. J. Pharmacol. 486, 31–34. doi: 10.1016/j.ejphar.2003.12.002
Valentine, G. W., Mason, G. F., Gomez, R., Fasula, M., Watzl, J., Pittman, B., et al. (2011). The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 191, 122–127. doi: 10.1016/j.pscychresns.2010.10.009
van Marwijk, H., Allick, G., Wegman, F., Bax, A., and Riphagen, I. I. (2012). Alprazolam for depression. Cochrane Database Syst. Rev. 7:CD007139. doi: 10.1002/14651858.CD007139.pub2
Varga, Z., Csabai, D., Miseta, A., Wiborg, O., and Czeh, B. (2017). Chronic stress affects the number of GABAergic neurons in the orbitofrontal cortex of rats. Behav. Brain Res. 316, 104–114. doi: 10.1016/j.bbr.2016.08.030
Viollet, C., Vaillend, C., Videau, C., Bluet-Pajot, M. T., Ungerer, A., L’Heritier, A., et al. (2000). Involvement of sst2 somatostatin receptor in locomotor, exploratory activity and emotional reactivity in mice. Eur. J. Neurosci. 12, 3761–3770. doi: 10.1046/j.1460-9568.2000.00249.x
Vithlani, M., Hines, R. M., Zhong, P., Terunuma, M., Hines, D. J., Revilla-Sanchez, R., et al. (2013). The ability of BDNF to modify neurogenesis and depressive-like behaviors is dependent upon phosphorylation of tyrosine residues 365/367 in the GABA(A)-receptor gamma2 subunit. J. Neurosci. 33, 15567–15577. doi: 10.1523/JNEUROSCI.1845-13.2013
Vollenweider, I., Smith, K. S., Keist, R., and Rudolph, U. (2011). Antidepressant-like properties of alpha2-containing GABA(A) receptors. Behav. Brain Res. 217, 77–80. doi: 10.1016/j.bbr.2010.10.009
Wang, D. S., Penna, A., and Orser, B. A. (2017). Ketamine increases the function of gamma-aminobutyric acid type A receptors in hippocampal and cortical neurons. Anesthesiology 126, 666–677. doi: 10.1097/ALN.0000000000001483
Wang, X., Dow-Edwards, D., Keller, E., and Hurd, Y. L. (2003). Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118, 681–694. doi: 10.1016/S0306-4522(03)00020-4
Wedzony, K., and Chocyk, A. (2009). Cannabinoid CB1 receptors in rat medial prefrontal cortex are colocalized with calbindin- but not parvalbumin- and calretinin-positive GABA-ergic neurons. Pharmacol. Rep. 61, 1000–1007. doi: 10.1016/S1734-1140(09)70161-6
Westrin, A., Ekman, R., and Traskman-Bendz, L. (1999). Alterations of corticotropin releasing hormone (CRH) and neuropeptide Y (NPY) plasma levels in mood disorder patients with a recent suicide attempt. Eur. Neuropsychopharmacol. 9, 205–211. doi: 10.1016/S0924-977X(98)00026-1
Widdowson, P. S., Ordway, G. A., and Halaris, A. E. (1992). Reduced neuropeptide Y concentrations in suicide brain. J. Neurochem. 59, 73–80. doi: 10.1111/j.1471-4159.1992.tb08877.x
Widerlov, E., Lindstrom, L. H., Wahlestedt, C., and Ekman, R. (1988). Neuropeptide Y and peptide YY as possible cerebrospinal fluid markers for major depression and schizophrenia, respectively. J. Psychiatr. Res. 22, 69–79. doi: 10.1016/0022-3956(88)90030-1
Wilkinson, S. T., and Sanacora, G. (2018). A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov. Today doi: 10.1016/j.drudis.2018.11.007 [Epub ahead of print].
Workman, E. R., Haddick, P. C., Bush, K., Dilly, G. A., Niere, F., Zemelman, B. V., et al. (2015). Rapid antidepressants stimulate the decoupling of GABA(B) receptors from GIRK/Kir3 channels through increased protein stability of 14-3-3eta. Mol. Psychiatry 20, 298–310. doi: 10.1038/mp.2014.165
Workman, E. R., Niere, F., and Raab-Graham, K. F. (2013). mTORC1-dependent protein synthesis underlying rapid antidepressant effect requires GABABR signaling. Neuropharmacology 73, 192–203. doi: 10.1016/j.neuropharm.2013.05.037
Xiong, Z., Zhang, K., Ishima, T., Ren, Q., Chang, L., Chen, J., et al. (2018). Comparison of rapid and long-lasting antidepressant effects of negative modulators of alpha5-containing GABAA receptors and (R)ketamine in a chronic social defeat stress model. Pharmacol. Biochem. Behav. 175, 139–145. doi: 10.1016/j.pbb.2018.10.005
Yang, Y., Babygirija, R., Zheng, J., Shi, B., Sun, W., Zheng, X., et al. (2018). Central neuropeptide Y plays an important role in mediating the adaptation mechanism against chronic stress in male rats. Endocrinology 159, 1525–1536. doi: 10.1210/en.2018-00045
Yeung, M., Engin, E., and Treit, D. (2011). Anxiolytic-like effects of somatostatin isoforms SST 14 and SST 28 in two animal models ( Rattus norvegicus ) after intra-amygdalar and intra-septal microinfusions. Psychopharmacology 216, 557–567. doi: 10.1007/s00213-011-2248-x
Zadrozna, M., Nowak, B., Lason-Tyburkiewicz, M., Wolak, M., Sowa-Kucma, M., Papp, M., et al. (2011). Different pattern of changes in calcium binding proteins immunoreactivity in the medial prefrontal cortex of rats exposed to stress models of depression. Pharmacol. Rep. 63, 1539–1546. doi: 10.1016/S1734-1140(11)70718-6
Zanos, P., Moaddel, R., Morris, P. J., Georgiou, P., Fischell, J., Elmer, G. I., et al. (2016). NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486. doi: 10.1038/nature17998
Zanos, P., Nelson, M. E., Highland, J. N., Krimmel, S. R., Georgiou, P., Gould, T. D., et al. (2017). A negative allosteric modulator for alpha5 subunit-containing GABA receptors exerts a rapid and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro 4:ENEURO.285-ENEURO.216. doi: 10.1523/ENEURO.0285-16.2017
Zarate, C. A. Jr., Singh, J. B., Carlson, P. J., Brutsche, N. E., Ameli, R., Luckenbaugh, D. A., et al. (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864. doi: 10.1001/archpsyc.63.8.856
Zhou, W., Wang, N., Yang, C., Li, X. M., Zhou, Z. Q., and Yang, J. J. (2014). Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur. Psychiatry 29, 419–423. doi: 10.1016/j.eurpsy.2013.10.005
Zou, D., Chen, L., Deng, D., Jiang, D., Dong, F., McSweeney, C., et al. (2016). DREADD in parvalbumin interneurons of the dentate gyrus modulates anxiety, social interaction and memory extinction. Curr. Mol. Med. 16, 91–102. doi: 10.2174/1566524016666151222150024
Keywords : depression, stress, prefrontal cortex, GABA, somatostatin, parvalbumin, ketamine
Citation: Fogaça MV and Duman RS (2019) Cortical GABAergic Dysfunction in Stress and Depression: New Insights for Therapeutic Interventions. Front. Cell. Neurosci. 13:87. doi: 10.3389/fncel.2019.00087
Received: 15 January 2019; Accepted: 20 February 2019; Published: 12 March 2019.
Reviewed by:
Copyright © 2019 Fogaça and Duman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronald S. Duman, [email protected]
This article is part of the Research Topic
Stress Disrupts GABA Signaling: Relevance for Stress-Related Psychiatric Disorders
- Help & FAQ
GABAergic control of depression-related brain states
- Department of Biochemistry and Molecular Biology
- Department of Psychiatry and Behavioral Health
- Penn State Neuroscience Institute
Research output : Chapter in Book/Report/Conference proceeding › Chapter
The GABAergic deficit hypothesis of major depressive disorders (MDDs) posits that reduced γ-aminobutyric acid (GABA) concentration in brain, impaired function of GABAergic interneurons, altered expression and function of GABA A receptors, and changes in GABAergic transmission dictated by altered chloride homeostasis can contribute to the etiology of MDD. Conversely, the hypothesis posits that the efficacy of currently used antidepressants is determined by their ability to enhance GABAergic neurotransmission. We here provide an update for corresponding evidence from studies of patients and preclinical animal models of depression. In addition, we propose an explanation for the continued lack of genetic evidence that explains the considerable heritability of MDD. Lastly, we discuss how alterations in GABAergic transmission are integral to other hypotheses of MDD that emphasize (i) the role of monoaminergic deficits, (ii) stress-based etiologies, (iii) neurotrophic deficits, and (iv) the neurotoxic and neural circuit-impairing consequences of chronic excesses of glutamate. We propose that altered GABAergic transmission serves as a common denominator of MDD that can account for all these other hypotheses and that plays a causal and common role in diverse mechanistic etiologies of depressive brain states and in the mechanism of action of current antidepressant drug therapies.

Publication series
All science journal classification (asjc) codes.
- Pharmacology
Access to Document
- 10.1016/bs.apha.2014.11.003
Other files and links
- Link to publication in Scopus
- Link to the citations in Scopus
Fingerprint
- Major Depressive Disorder Medicine & Life Sciences 100%
- Depression Medicine & Life Sciences 63%
- Brain Medicine & Life Sciences 47%
- Antidepressive Agents Medicine & Life Sciences 34%
- Aminobutyrates Medicine & Life Sciences 28%
- GABA-A Receptors Medicine & Life Sciences 20%
- Chlorides Medicine & Life Sciences 20%
- Interneurons Medicine & Life Sciences 20%
T1 - GABAergic control of depression-related brain states
AU - Luscher, Bernhard
AU - Fuchs, Thomas
N1 - Publisher Copyright: © 2015 Elsevier Inc.
N2 - The GABAergic deficit hypothesis of major depressive disorders (MDDs) posits that reduced γ-aminobutyric acid (GABA) concentration in brain, impaired function of GABAergic interneurons, altered expression and function of GABAA receptors, and changes in GABAergic transmission dictated by altered chloride homeostasis can contribute to the etiology of MDD. Conversely, the hypothesis posits that the efficacy of currently used antidepressants is determined by their ability to enhance GABAergic neurotransmission. We here provide an update for corresponding evidence from studies of patients and preclinical animal models of depression. In addition, we propose an explanation for the continued lack of genetic evidence that explains the considerable heritability of MDD. Lastly, we discuss how alterations in GABAergic transmission are integral to other hypotheses of MDD that emphasize (i) the role of monoaminergic deficits, (ii) stress-based etiologies, (iii) neurotrophic deficits, and (iv) the neurotoxic and neural circuit-impairing consequences of chronic excesses of glutamate. We propose that altered GABAergic transmission serves as a common denominator of MDD that can account for all these other hypotheses and that plays a causal and common role in diverse mechanistic etiologies of depressive brain states and in the mechanism of action of current antidepressant drug therapies.
AB - The GABAergic deficit hypothesis of major depressive disorders (MDDs) posits that reduced γ-aminobutyric acid (GABA) concentration in brain, impaired function of GABAergic interneurons, altered expression and function of GABAA receptors, and changes in GABAergic transmission dictated by altered chloride homeostasis can contribute to the etiology of MDD. Conversely, the hypothesis posits that the efficacy of currently used antidepressants is determined by their ability to enhance GABAergic neurotransmission. We here provide an update for corresponding evidence from studies of patients and preclinical animal models of depression. In addition, we propose an explanation for the continued lack of genetic evidence that explains the considerable heritability of MDD. Lastly, we discuss how alterations in GABAergic transmission are integral to other hypotheses of MDD that emphasize (i) the role of monoaminergic deficits, (ii) stress-based etiologies, (iii) neurotrophic deficits, and (iv) the neurotoxic and neural circuit-impairing consequences of chronic excesses of glutamate. We propose that altered GABAergic transmission serves as a common denominator of MDD that can account for all these other hypotheses and that plays a causal and common role in diverse mechanistic etiologies of depressive brain states and in the mechanism of action of current antidepressant drug therapies.
UR - http://www.scopus.com/inward/record.url?scp=84921691547&partnerID=8YFLogxK
UR - http://www.scopus.com/inward/citedby.url?scp=84921691547&partnerID=8YFLogxK
U2 - 10.1016/bs.apha.2014.11.003
DO - 10.1016/bs.apha.2014.11.003
M3 - Chapter
C2 - 25637439
AN - SCOPUS:84921691547
T3 - Advances in Pharmacology
BT - Advances in Pharmacology
PB - Academic Press Inc.
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (44,004,653 articles, preprints and more)
- Free full text
- Citations & impact
- Similar Articles
The GABAergic deficit hypothesis of major depressive disorder.
Author information, affiliations.
- Luscher B 1
ORCIDs linked to this article
- Luscher B | 0000-0003-1375-1906
Molecular Psychiatry , 16 Nov 2010 , 16(4): 383-406 https://doi.org/10.1038/mp.2010.120 PMID: 21079608 PMCID: PMC3412149
Free full text in Europe PMC
Abstract
Free full text .

The GABAergic Deficit Hypothesis of Major Depressive Disorder
Bernhard luscher.
1 Departments of Biology, Pennsylvania State University, University Park, PA 16802
2 Departments of Biochemistry & Molecular Biology, Pennsylvania State University, University Park, PA 16802
3 Department of Psychiatry, Pennsylvania State University, College of Medicine, Hershey, PA 17033
4 Center for Molecular Investigation of Neurological Disorders, Pennsylvania State University, University Park, PA 16802
Qiuying Shen
Nadia sahir.
Increasing evidence points to an association between major depressive disorders (MDDs) and diverse types of GABAergic deficits. Here we summarize clinical and preclinical evidence supporting a central and causal role of GABAergic deficits in the etiology of depressive disorders. Studies of depressed patients indicate that MDDs are accompanied by reduced brain concentration of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) as well as alterations in the subunit composition of the principal receptors (GABA A receptors) mediating GABAergic inhibition. In addition, there is abundant evidence that GABA plays a prominent role in the brain control of stress, the most important vulnerability factor in mood disorders. Furthermore, preclinical evidence suggests that currently used antidepressant drugs designed to alter monoaminergic transmission as well as non-pharmacologic therapies may ultimately act to counteract GABAergic deficits. In particular, GABAergic transmission plays an important role in the control of hippocampal neurogenesis and neural maturation, which are now established as cellular substrates of most if not all antidepressant therapies. Lastly, comparatively modest deficits in GABAergic transmission in GABA A -receptor-deficient mice are sufficient to cause behavioral, cognitive, neuroanatomical, and neuroendocrine phenotypes as well as antidepressant drug response characteristics expected of an animal model of MDD. The GABAergic hypothesis of MDD suggests that alterations in GABAergic transmission represent fundamentally important aspects of the etiological sequelae of major depressive disorders that are reversed by monoaminergic antidepressant drug action.
- Introduction
Major depressive disorder (MDD) represents a complex neuropsychiatric syndrome with a lifetime prevalence of approximately 17% of the population worldwide 1 . It exhibits high comorbidity with anxiety disorders, with 50–60% of depressed patients reporting a lifetime history of anxiety disorders, and many anxiety disorder patients showing a history of treatment for depression 2 – 9 . Antidepressant drug (AD) treatments currently in use for both anxiety and depressive disorders are designed to target monoaminergic neurotransmission, and they have set the foundation for the so-called catecholamine 10 , 11 and serotonin 12 , 13 hypotheses of affective disorders. Collectively, these hypotheses posit that antidepressants act by increasing the extracellular concentration and function of monoamine transmitters in the forebrain 14 and, by extension, that mood disorders are caused by altered production, release, turnover, or function of monoamine transmitters or altered function of their receptors. There is, however, a growing consensus that altered monoaminergic transmission is insufficient to explain the etiology of depressive disorders 15 and that currently used antidepressants instead are modulating other neurochemical systems that have a more fundamental role in MDD 16 .
A more recent hypothesis suggests that depressive disorders represent stress disorders. It is supported by a large body of epidemiological evidence showing that stress is a major vulnerability factor for mood disorders 17 – 19 . This evidence includes altered HPA axis function in patients 20 , 21 , polymorphisms in the CRH1 (corticotropin releasing hormone 1) receptor gene that are associated with mood disorders 22 , as well as data from rodents showing that central administration of stress-related hormones can produce pathologies reminiscent of MDD, which are reversed by antidepressant drug treatment 23 , 24 . An extension of the stress hypothesis puts forward that depressive disorders are caused by inadequate trophic support of neurons and impaired neural plasticity 25 – 28 . None of the current hypotheses, however, have identified a unified molecular framework that is broadly implicated in the etiology of mood disorders and antidepressant drug mechanisms.
Here we summarize older but underreported and recent or emerging evidence in support of a fourth hypothesis that posits that etiological origins of mood disorders converge on genetic, epigenetic or stress-induced deficits in GABAergic transmission as a principal cause of MDDs, and that the therapeutic effects of currently used monoaminergic antidepressants involve downstream alterations in GABAergic transmission.
- GABA and its receptors
GABA A receptors vs. GABA B receptors
GABA is the principal neurotransmitter mediating neural inhibition in the brain. GABAergic neurons are present throughout all levels of the neuraxis, represent between 20 and 40% of all neurons depending on brain region, and are known to balance and fine tune excitatory neurotransmission of various neuronal systems including the monoaminergic and cholinergic projections to the forebrain. GABA exerts its effects by activation of two entirely different classes of receptors, the ionotropic GABA A receptors (GABA A Rs) and the metabotropic GABA B Rs. GABA A Rs are known as key control elements of anxiety state based on the potent anxiolytic activity of benzodiazepines (BZs) that act as positive allosteric modulators of a major subset of GABA A Rs. Accumulating evidence described below points to marked alterations in GABA A R signaling in both anxiety and mood disorders. GABA B Rs are members of the G-protein coupled receptor family and they have been recently implicated in affective disorders based on altered anxiety- and depression-related behavioral measures in mice subject to pharmacological and genetic manipulations of these receptors. GABA B (1) and GABA B (2)R KO mice show behavior indicative of increased anxiety combined with an antidepressant phenotype 29 , 30 . Consistent with these genetic studies, positive GABA B R modulators show potential as anxiolytics, whereas antagonists have antidepressant-like effects in animal experiments 29 . However, given the strong evidence for comorbidity of anxiety and depressive disorders, opposing actions of GABA B -directed ligands on anxiety- and depression-related measures are likely to limit the potential of GABA B R-directed therapeutic approaches. Therefore, in this review we will focus on GABA signaling through GABA A Rs, the receptors that mediate the vast majority of GABA function.
Structure of GABA A Rs
Subunit composition.
Structurally, GABA A Rs represent heteropentameric GABA-gated chloride channels that are assembled from subunits encoded by 19 different genes (α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3). Different combination of these subunits give rise to a large number of structurally, functionally and pharmacologically distinct receptor subtypes, of which about 25 have been either definitely or tentatively identified 31 . These can be roughly subdivided into i) postsynaptic and ii) extra- or perisynaptic subtypes, although some neurons also contain GABA A Rs at axon terminals. The postsynaptic GABA A R subtypes include mainly the α1βγ2, α2βγ2, and α3βγ2 receptors whose β subunit remain ill defined; they tend to be concentrated at synapses where they mediate phasic inhibitory synaptic currents in response to synaptically released GABA. The latter consist of α4βδ and α5βγ2 receptors in forebrain and α6βδ in cerebellum. They are located on somatodendritic membrane compartments away from the synaptic cleft and tonically activated by low ambient concentrations of GABA or GABA spilled over from synapses 31 , 32 .
Functional dissociation of different subtypes of BZ-sensitive GABA A Rs
BZs act as positive allosteric modulators of GABA A Rs composed of α1βγ2, α2βγ2, α3βγ2, or α5βγ2 subunits. Using a combined molecular genetic and behavioral pharmacologic strategy these GABA A R subtypes have been assigned to different diazepam-sensitive behaviors based on the specific type of α subunit present 33 , 34 . In particular, it was found that the broadly expressed α1βγ2 receptor subtype mediates sedative, anterograde amnesic, addictive and most of the anticonvulsant effects of diazepam 35 – 38 . In contrast, α2βγ2 receptors control the anxiolytic and anti-hyperalgesic properties 39 , 40 , and α2βγ2, α3βγ2, and α5βγ2 receptors together mediate the myorelaxant effects of diazepam 41 , 42 . The α5βγ2 receptors are further important for normal hippocampus-dependent associative memory functions and for the development of tolerance to the sedative functions of diazepam 42 – 45 . The prevalent distribution of α2βγ2 receptors in the cerebral cortex, hippocampus, and amygdala 46 and the role of this receptor subtype in anxiolysis is consistent with the established role of corticolimbic brain regions in the control of emotional states 47 , 48 . Moreover, the identification of α1βγ2 receptorsin interneurons of the ventral tegmental area (VTA) as substrates for the addictive properties of BZs 37 suggests that functional deficits of these receptors may contribute to anhedonia as seen in GABA A R γ2 subunit-deficient mice 49 (see below). Functional deficits in α1βγ2 receptors can be predicted to increase GABA release by VTA interneurons and to enhance GABAergic inhibition of nearby dopaminergic neurons, and thereby to contribute to anhedonia as a core symptom of major depressive disorder.
BZ insensitive GABA A Rs
In contrast to most postsynaptic γ2-containing GABA A Rs, the extrasynaptic receptor subtypes composed of α4βδ subunits in the forebrain and α6βδ subunits in the cerebellum are insensitive to the GABA-potentiating effects of BZs, and they conduct a prominent tonic form of inhibition. Nevertheless, they exhibit high affinity for the imidazo-BZ Ro15-4513 and flumazenil, as well as the iodinated flumazenil derivative [ 123 I]iomazenil 50 – 52 . These receptors therefore are included along with BZ-sensitive GABA A Rs in autoradiographic and nuclear tomographic measurements using these ligands. The α4βδ receptors are of increasing interest as they are dynamically regulated by stress and other hormonal stimuli implicated in mood disorders.
- Brain imaging studies suggest a role for altered GABAergic transmission in anxiety and depressive disorders
GABA deficits in depression
The strongest evidence that GABAergic deficits may contribute to depressive disorders is based on reduced GABA levels in plasma 53 , 54 and cerebrospinal fluid 55 or resected cortical tissue 56 of depressed patients. While initial findings were controversial 57 or lacked statistical significance 58 , more recent assessments of GABA deficits in brain using proton magnetic resonance spectroscopy show dramatic reductions of GABA in the occipital cortex 59 , 60 and lower but still significant reductions in the anterior cingulate and dorsomedial/dorsolateral prefrontal cortex 61 , 62 of MDD patients. This neurochemical phenotype is consistent with a selective loss of calbindin positive GABAergic interneurons observed in the dorsal prefrontal cortex of depressed patients 63 . Interestingly, GABA deficits are most pronounced in melancholic and treatment-resistant subtypes of depression (−50%) 56 , 60 , 64 , while reductions in depressed patients not meeting criteria of melancholia 60 and in bipolar patients 65 are less severe (−20%).
GABA A R deficits in anxiety disorders
Reduced abundance of GABA A R binding sites suggests a role for GABAergic deficits in anxiety disorders. Positron Emission Tomography (PET) scanning using the BZ site antagonist 11 C-flumazenil shows global reductions in GABA A R binding sites in patients suffering from panic attacks, with the most robust changes in ventral basal ganglia, orbitofrontal and temporal cortex 66 , which are thought to control the experience of anxiety 67 , 68 . Moreover, while flumazenil has no behavioral effect in healthy people, it precipitates panic attacks during symptom free episodes in panic patients, suggesting unusual inverse agonist properties 69 . Analyses by Single Photon Emission Computed Tomography (SPECT) with a similar ligand ([ 123 I]iomazenil) show widespread reductions in GABA A R binding sites in the superior frontal, temporal, and parietal cortex 70 , left hippocampus and precuneus 71 of panic patients. Similar analyses have revealed GABA A R deficits in the temporal lobe of patients with generalized anxiety disorder 72 and medial prefrontal cortex of patients suffering from posttraumatic stress disorder 73 . Collectively, the data suggest that different anxiety disorders involve GABA A R deficits in different brain regions.
Gene expression changes associated with major depressive disorder suggest altered expression and subunit composition of GABA A Rs
In contrast to anxiety disorders, the density of GABA A R [ 123 I]iomazenil binding sites in brain of depressed subjects is largely unchanged 74 . A notable exception is a single patient suffering from severe treatment-resistant anxious depression with panic attacks linked to a silent point mutation in the GABA A R β1 subunit gene 75 . However, there is abundant evidence for a role of GABA A Rs in major depression based on altered expression of GABA A R subunit transcripts ( Table 1 ). A genome wide screen for changes in transcript levels in the frontopolar cortex [Brodmann area (BA)10] of suicide victims that had suffered from various forms of depressive disorders has revealed reductions in the abundance of α1, α3, α4 and δ subunit mRNAs 76 . Evidence for similarly discoordinated expression of GABA A R subunit transcripts is also available for other brain areas implicated in mood disorders 77 . These studies did not differentiate among changes linked to depression, suicide, or suicide-associated distress, and thus need to be confirmed in a more representative cohort of patients and controls. Interestingly, the reduced expression of the α1 mRNA was associated with increased DNA methylation of transcriptional control regions of the GABRA1 gene and with upregulated expression of the DNA methyltransferase DNMT-3B, suggesting that GABRA1 gene expression is subject to epigenetic control 78 .
Depression related alterations in expression of GABA A R subunit genes.
BA4, motor cortex; BA6, supplementary motor area (medial) and premotor cortex (lateral); BA9/44/46, dorsolateral prefrontal cortex; BA10, frontopolar cortex; BA20, Inferior temporal gyrus; BA21 middle temporal area; BA24, anterior cingulate cortex; BA38 temporopolar area; BA47, ventrolateral prefrontal cortex.
A comparison of postmortem brains of depressed vs. non-depressed suicide victims has revealed increased expression of the α5, γ2, and δ subunit mRNAs in the dorsolateral prefrontal cortex (BA44/46) 79 . This is consistent with an earlier report showing upregulation of β3, γ2 and δ subunit mRNAs in similar brain regions (BA9, 46) of depressed patients who died from more diverse causes 80 . This latter study has further identified selective upregulation of α5 mRNA in the anterior cingulate cortex (BA24), a critical component of the corticolimbic pathway affected in major depression 81 . A comprehensive screen for gene expression changes in 17 cortical and subcortical brain regions from depression-related suicides found that genes that are involved in GABAergic transmission are among the most consistently changed 82 . Among a total of 27 GABAergic probe sets differentially expressed in the frontal cortex or hippocampus no fewer than 19 involve genes that encode GABA A R subunits. GABA A R subunit genes are mostly upregulated in depression-related suicides, perhaps as a compensatory mechanism for low GABA levels associated with depression. Low levels of GABA A R gene expression among suicides that lack a history of depression suggest that elevated expression in depression-related suicides may in fact be depression-specific 82 . These increases in GABA A R subunit mRNAs seem to contradict the aforementioned unaltered levels of GABA A R binding sites 74 in suicide brains. However, altered subunit mRNA levels do not necessarily have to result in changes in GABA A R binding sites, neither of which are representative of functional receptors present at the plasma membrane or at synapses. Discoordinated expression of GABA A R subunits might give rise to functionally distinct GABA A R subtypes that nevertheless bind [ 123 I]iomazenil. Lastly, GABA A Rs are subject to phosphorylation, palmitoylation and ubiquitination, all of which regulate the cell surface expression and accumulation of GABA A Rs at synapses, as well as inhibitory synaptogenesis 83 , 84 . These posttranslational modifications allow for modulation of GABA A R cell surface expression by environmental and physiological cues implicated in mood disorders. Accordingly, mutations in trafficking proteins that regulate the portion of GABA A Rs at synapses affect anxiety and mood-related behavior in both patients 85 and animal models 86 , 87 .
- Genetic evidence in support of GABAergic deficits in mood disorders
There is growing evidence that genetic polymorphisms in GABA A R subunit genes are involved in affective disorders. The Wellcome Trust Case Control Consortium has identified a strong association between bipolar disorder (BPD) and polymorphism in the GABRB1 gene coding for the β1 subunit of GABA A Rs 88 . A follow-up study has confirmed this finding and extended it to associations with nucleotide polymorphisms in the GABRA4, GABRB3, GABRA5 and GABRR1 subunit genes 89 . Notably, GABRB1, GABRA4, and GABRR1 are part of the same gene cluster on chromosome 4p12, together with GABRA2, while GABRA5 and GABRB3 are part of a cluster at 15q11-q13, which had previously been implicated in BPD 90 . Associations between nucleotide polymorphisms and BPD further exist for GABRA3 91 and GABRB2 92 , with the latter implicated in alternative splicing of the β2 subunit mRNA 93 . For MDD, genetic associations have been described for GABRA5 94 and the gene cluster encoding GABRA1 95 , 96 , GABRA6 and GABRG2 96 . Although not all studies have found this latter association 97 , this same gene cluster is linked to depression-related behavior also in mice 98 . Finally, there is recent evidence for a male-specific association between non-coding genetic polymorphisms of the GABRD gene and childhood-onset mood disorders 99 . In summary, the data suggest that GABAergic deficit can lead to mood disorders but also demonstrate that genetic polymorphisms at the level of GABA A R subunit genes account for at most a small percentage of mood disorders, and that environmental and remote genetic triggers of GABAergic deficits may be more important.
Modulation of GABA A Rs by stress: a major risk factor of depressive disorders
Effects of early life stress.
Stress represents the most important vulnerability factor for MDD and related neuropsychiatric disorders, both in the developing 100 – 104 and adult nervous system 105 . There is a growing body of preclinical evidence that much of this vulnerability may be due to stress-induced impairment of GABAergic transmission. For example, maternal separation stress of rats during the first postnatal weeks leads to increased neophobia and acoustic startle responses in adulthood, and this phenotype is associated with reduced expression of BZ-sensitive GABA A Rs in the frontal cortex, amygdala, locus coeruleus and the n. tractus solitarius 106 . The level of maternal care measured in the form of pup licking in rodents is positively correlated with GABA A R mRNA expression and inversely related to behavioral stress reactivity in adulthood 107 . Analyses of GABA A R γ2-deficient mice 49 , 108 (further discussed below) suggest that modest reductions in GABA A R function during development are not just correlated with anxiety- and depression-related behavior in adulthood, but that they can be causal.
Effects of stress in adulthood
In addition to early life stress effects on GABA A R expression in the mature brain, there is an extensive literature on stress-induced changes in the expression and function of GABA A Rs in the adult brain. The exact consequences of acute stress on GABA A R expression in rodents appear to depend on the type of stress protocol, sex and brain region(s) analyzed 109 . Most relevant in the context of this review, however, are unpredictable chronic forms of stress that are suitable to model depressive-like symptoms in animal models 110 , 111 . The prevalent effect of chronic stress in the cerebral cortex is reduced abundance and function of GABA A Rs 112 . By contrast, the effects of chronic stress hormone exposure in the hippocampus are uneven and subunit- and layer-specific 113 , 114 . In particular, expression of α4βδ receptors is subject to prominent chronic stress-induced augmentation in granule and pyramidal cell neurons of the hippocampus 115 , 116 . This chronic effect is thought to alter sensitivity of the brain to acute stress-associated increases in neuroactive steroids, as discussed further below.
GABAergic control of HPA axis
Increased secretion of glucocorticoids and aberrant function of the hypothalamic–pituitary–adrenal (HPA) axis are well-replicated findings in a major subset of patients suffering from severe forms of depressive disorders, especially melancholic depression 19 , 21 , 117 – 120 ( Figure 1 ). The paraventricular nucleus (PVN) of the hypothalamus, which is the source of corticotropin-releasing hormone (CRH) that dictates HPA axis responses to stress 121 – 123 , is subject to GABAergic inhibitory control by frontal cortex 122 , 124 and ventral hippocampus 125 . They are activated along with the PVN in response to acute emotional stress 126 and represent major sites of vulnerability to stress 127 – 130 .

The GABAergic deficit hypothesis of MDD presented here suggests that local GABAergic deficits in hippocampus and frontal cortex due to reduced GABA release, uncoordinated GABA A R subunit gene expression or anomalous signaling mechanisms that affect GABA A R accumulation at the plasma membrane lead to local hyperexcitability, which is relayed by projections (In the case of frontal cortex through the BNST 144 ) to the PVN of the hypothalamus. In the hippocampus such local GABAergic deficits may involve loss of parvalbumin positive interneurons 131 , reduced GABAergic synaptic inhibition 130 and reduced maturation and survival of adult-born granule cells 108 , which is sufficient to activate the HPA axis 135 . Cortical deficits in GABAergic inhibition include reduced GABA levels in patients 61 , 62 . In addition, GABAergic deficits may be induced by chronic stress, which down-regulates the expression and function of GABA A Rs in the frontal cortex 112 . Hyperexcitability of the cortex and hippocampus is relayed by projections to the PVN. Local GABAergic inhibition of PVN neurons may be independently compromised by a stress-induced shift in the neural Cl − reversal potential 147 . The ensuing excessive release of CRH from the PVN results in increased release of ACTH from the anterior pituitary, which promotes the release of glucocorticoids, thereby closing a positive feedback loop that amplifies cortical and hippocampal GABAergic deficits. Adrenal neurosteroids normally potentiate GABA-mediated activation of GABA A Rs on dentate gyrus granule cells 168 , 381 . Moreover THDOC upregulates the expression of α4βδ receptors in hippocampal granule cells 115 . However, in CA1 pyramidal cells of the hippocampus the same neurosteroids facilitate GABA-induced desensitization of α4βδ receptors 153 , which increases neural excitability 168 .
In contrast to acute stress, which enhances GABAergic synaptic transmission in the ventral hippocampus 130 , chronic stress causes reductions in GABAergic synaptic currents due to the selective loss of hippocampal parvalbumin-positive interneurons 131 . This effect has been attributed to glucocorticoids acting on a membrane-bound, ill-defined receptor that evokes NO release from hippocampal pyramidal cells 131 . Even modest chronic deficits in GABAergic transmission in GABA A R γ2 +/− mice impair the survival of adult-born hippocampal neurons 108 , an effect that may explain hippocampal volume reductions seen in chronically depressed patients 132 – 134 (see also below). Blocking hippocampal neurogenesis in turn is sufficient to increase HPA axis activity 135 . Thus, projections from the ventral hippocampus via the lateral septum 128 , 136 to the hypothalamus link hippocampal neuropathology to hyperactivity of the HPA axis and aberrant stress reactivity, which may sustain or even amplify hippocampal neuropathology.
Similar to the hippocampus, the dorsomedial and dorsolateral prefrontal and the anterior and subgenual cingulate cortices represent substrates of stress-related psychiatric illness associated with cognitive and affective symptoms of MDD 81 , 129 , 137 – 139 . The deficits in cortical GABA concentrations 61 , 62 and altered expression of GABA A R subunit genes ( Table 1 ) indicate that this phenotype involves reduced GABAergic function. In addition, cortical GABAergic inhibition is impaired by stress-induced signaling pathways, as indicated by drastic CRH-induced, serotonin-mediated desensitization of GABAergic inhibitory synaptic currents recorded from cortical slices 140 . Tracing experiments show that GABAergic neurons of the anterior bed nucleus of the stria terminalis (BNST) serve to relay inhibitory control by the medial prefrontal cortex to the PVN 141 – 144 . Moreover, mice with genetically-induced cortex/hippocampus-restricted GABA A R deficits exhibit chronically elevated HPA axis activity 49 . Thus, local cortical deficits in GABAergic inhibition and correspondingly increased neural excitability lead to increased activity of the PVN, even if the initially causal deficit is limited to extra-hypothalamic circuits (see also below).
In addition to remote inhibition of the hypothalamus by cortical and hippocampal GABAergic circuits, CRH-producing neurons of the PVN themselves are subject to local GABAergic inhibitory control that is regulated by stress 145 . Chronic mild stress of rats results in a marked reduction of the frequency but unaltered amplitude of GABAergic inhibitory synaptic currents recorded from PVN neurons, suggesting presynaptic deficits in GABA release 146 . However, postsynaptic GABAergic function of PVN neurons is also impaired, as indicated by stress-induced down-regulation of the K + -Cl − co-transporter KCC2. The ensuing depolarizing shift of the chloride reversal membrane potential renders GABA inputs ineffective, thereby leading to increased excitability of PVN neurons 147 . Increased CRH release by PVN neurons leads to increased release of adrenocorticotropic hormone (ACTH) by the anterior pituitary gland and systemically elevated basal cortisol levels (corticosterone in rodents) and other stress hormones, which are well-replicated findings in prominent subsets of patients suffering from severe forms of depressive disorders 19 , 117 – 120 , 148 ( Figure 1 ).
GABA A R modulation by neurosteroids
Stress is known to affect GABAergic inhibition at least in part through stress-induced release of endogenous neuroactive steroids that act as allosteric modulators of GABA A Rs. In particular, 3α,5α-tetrahydroprogesterone (THP, also known as [allo]pregnanolone) and 3α,21-dihydroxy-5α-pregnan-20-one (THDOC, [allo]tetrahydrodeoxycorticosterone) are rapidly induced (4 – 20 fold) by stress 149 and known to act as high-affinity modulators of extrasynaptic α4βδ GABA A Rs 150 – 152 . THP either increases (in dentate gyrus granule cells) or reduces (in CA1 pyramidal cells) α4βδ receptor-mediated tonic GABAergic inhibition, due to cell type-specific differences in chloride homeostasis and steroid-induced receptor desensitization, which depends on the direction of the chloride gradient 152 , 153 . Preclinical and clinical data indicate that plasma concentrations of THP and THDOC are reduced and increased, respectively in depressed patients 154 – 157 and normalized by certain ADs (see below), which points to a role for neurosteroid synthesis in the pathology of depressive disorders. While THP is an endogenous metabolite of ovarian/adrenal progesterone and also produced in brain, THDOC is derived exclusively from adrenal sources 149 , 158 , 159 . Normally, α4βδ receptors are readily detectable only in dentate gyrus granule cells, most of the thalamus, striatum, pons, and in the outer layers of cerebral cortex 160 . However, prominent tonic inhibitory currents with a pharmacological profile of δ-containing GABA A Rs in PVN neurons 161 and attenuation of ACTH and corticosterone release by THP and THDOC 162 , 163 indicate that α4βδ receptors also contribute to the inhibitory control of HPA axis activity in the PVN.
The expression of α4βδ receptors is dynamically regulated
In CA1 pyramidal cells the accumulation of these receptors is strongly induced upon progesterone withdrawal 164 – 166 , at puberty 167 , 168 and during pregnancy 166 . In dentate granule cells the abundance of α4βδ receptors is subject to dynamic fluctuations across the ovarian cycle 169 , during pregnancy 166 , 170 , 171 , and induced by stress 115 . Thus, aberrant homeostatic regulation of neurosteroid synthesis together with cell type-specific effects on expression and function of α4βδ receptors is implicated in the etiology of stress-associated mood disorders, premenstrual dysphoric disorder (PMDD) and postpartum depression (PPD) 150 , 151 , 172 , 173 (see below).
- Pharmacologic evidence in support of a role of GABAergictransmission in depressive disorders
Antidepressant efficacy of benzodiazepines
A possible role of GABA A R dysregulation in mood disorders has been controversial in part due to lack of a consensus about whether BZs are therapeutically effective for the treatment of depression 61 . However, the limited use or efficacy of BZs in AD therapies should not be taken as evidence that GABAergic deficits are not involved in the etiology of MDD. Early studies concluded that standard tricyclic antidepressants (TCAs) are overwhelmingly superior to BZs, although the two classes of drugs were initially prescribed for depression almost interchangeably 174 . Indeed, some early studies reported antidepressant efficacy of BZs that was comparable to that of standard antidepressants 175 – 177 , with some studies reporting more rapid therapeutic onset 178 , 179 or greater efficacy of BZs 180 . More recent meta-analyses of clinical data have concluded that antidepressant efficacy of BZs is limited to the triazolo-BZ alprazolam, with classical BZs being ineffective beyond their established role as anxiolytics 181 , 182 . Alprazolam has been rated as equivalent or superior to TCAs with respect to anxiety and sleep indices of depression, equivalent with respect to improving anergia, psychomotor retardation and anhedonia, but inferior in relieving depressed mood 181 , 182 . The most obvious limitations to therapeutic efficacy of BZs are due to rapid development of tolerance, the high risk for developing dependence, the moderate abuse potential, and ultimately the danger of withdrawal symptoms 183 , 184 . At the cellular level, BZs may limit the proliferation of progenitors of adult-born hippocampal neurons, which would limit the effect these drugs can have on immature neurons, which act as a substrate of antidepressant drug action (see below). Nevertheless, BZs are often used in combination with standard antidepressants, even today, both for initial treatment and maintenance therapy 185 , 186 , which suggests beneficial effects. Encouragingly, the sedative hypnotic agent eszopiclone, which acts as a positive allosteric agonist similar to BZs but selectively on α2βγ2 and α3βγ2 subtypes of GABA A Rs, shows significant promise as an antidepressant in patients suffering from insomnia 187 – 189 .
GABAergic mechanisms of monoaminergic antidepressants
With the exception of some BZs mentioned above, currently used antidepressants exclusively target monoamine transmitters. They are designed to block the reuptake of extracellular serotonin (selective serotonin reuptake inhibitors, SSRIs), norepinephrine or, to a lesser extent, dopamine, or they unspecifically inhibit the intracellular degradation of monoamine transmitters. AD-induced increases in extracellular monoamines are thought to result in slow neurochemical, transcriptional, translational, posttranslational, and epigenetic adaptations that underlie therapeutically effective neural plasticity 28 . However, the receptors that mediate the functionally relevant neural adaptations of drug-induced increases in monoamine transmitters and their cellular localization have not been conclusively determined. Indeed, there is evidence that antidepressants may activate G-protein signaling independently of increased monoamine transmitters 190 , 191 . Even so, the antidepressant effects of serotonin in forebrain are thought to involve 5-HT1AR-mediated hyperpolarization of pyramidal cells 192 and 5-HT1B/5-HT2/5-HT3/5-HT4R-mediated excitation of GABAergic interneurons 193 – 197 . In support of this conclusion, the 5-HTR trafficking factor P11/S100A10 interacts with and regulates the cell surface expression and function of 5-HT1B 198 and 5-HT4Rs 199 . Electroconvulsive therapy (ECT) and chronic treatment with imipramine result in upregulation of P11 mRNA and protein selectively in the forebrain 198 . Moreover, P11 is required for normal antidepressant and neurogenic effects of fluoxetine 197 . Importantly, P11 is selectively expressed in several classes of hippocampal GABAergic interneurons but absent in granule cell precursors 197 . Thus, the effects of fluoxetine, imipramine and ECT may have in common that they involve increased excitability of GABAergic interneurons, which, in turn, can be predicted to increase GABAergic activation of hippocampal granule cell precursors 200 , 201 . Whereas GABAergic input to mature neurons is mostly hyperpolarizing, the depolarizing action of GABA on immature granule cells is implicated in the mechanism of monoaminergic AD action (see below).
AD-induced potentiation of GABA release as a mechanism underlying AD effects is congruent with chronic SSRI-mediated increases in cortical GABA concentrations observed in patients 202 and healthy volunteers 203 . However, these reports seem at odds with fluoxetine effects on GABA signaling in the visual cortex of rats 204 . Chronic fluoxetine-induced reductions in cortical GABA concentrations and correspondingly reduced GABAergic inhibition have been shown to reactivate ocular dominance plasticity in the adult brain and to promote the recovery of visual functions in adult amblyopic animals 204 . It remains to be seen whether such effects can be replicated with other antidepressants and whether they extend to brain areas implicated in mood disorders.
Similar to SSRIs, TCAs that increase the extracellular concentration of noradrenalin as well as 5-HT are likely to act in part by modulating GABAergic transmission. Noradrenergic innervation of GABAergic interneurons increases GABAergic transmission in diverse forebrain regions as shown for the frontal 205 , sensorimotor 206 and entorhinal cortices 207 , the CA1 hippocampus 208 and the basolateral amygdala 209 . The selective norepinephrine reuptake inhibitor reboxetine has complex brain region-specific effects on expression of interneuronal glutamic acid decarboxylase 67 (GAD67), the principal enzyme involved in the synthesis of GABA 210 . Immunostaining for GAD67 in brain of medication free depressed suicides is significantly reduced, whereas brain of a different cohort of depressed suicide victims who had been treated with SSRIs or TCAs showed normal levels of GAD67 211 . Collectively, the data suggest that norepinephrine and serotonin reuptake inhibitors have in common that they potentiate GABAergic transmission.
Direct effects of ADs on GABA A Rs
In addition to their principal effects on monoamine transporters and receptors, many if not all antidepressants can directly act on other targets that contribute to therapeutic efficacy, undesirable side effects, or toxicity upon overdose. For example, fluoxetine (1–10 μM) has direct off-target effects at nicotinic acetylcholine 212 , 213 and 5-HT3 receptors 214 – 216 as well as diverse Cl − 217 , voltage-gated Ca 2+ and K + channels 218 – 223 . Importantly, therapeutically relevant concentrations of fluoxetine and its metabolite norfluoxetine act as potent positive allosteric modulators of GABA A Rs in vitro when tested on receptors expressed in heterologous cells 224 and in cultured neurons 225 . This effect may not only contribute to antidepressant efficacy but also explain the unique anticonvulsant properties of fluoxetine in patients 226 .
AD-induced potentiation of GABAergic transmission by neurosteroids
Low concentrations of chronically applied fluoxetine or its active metabolite norfluoxetine and their relatives (i.e. paroxetine, fluvoxamine, sertraline) have been shown to increase the plasma or cerebrospinal fluid (CSF) concentrations of THP 155 – 157 , 227 – 230 . This effect is observed at concentrations fifty-times lower than the concentration that affects 5-HT uptake. Thus, THP appears to contribute to the anxiolytic function of SSRIs 231 . The behavioral effects of THP are independent of an increase in serotonin but are attenuated by bicucullin 232 , which shows that they involve potentiation of GABA A Rs. In vitro experiments with fluoxetine, sertraline, and paroxetine suggest that SSRI-induced increases in THP are due to direct drug effects on enzymes involved in THP synthesis 233 . Hippocampal administration of THP in rats has anxiolytic and antidepressant-like behavioral effects and is associated with increased expression of the γ2 subunit mRNA of GABA A Rs 234 . In addition to genomic effects, THP acts as a potent positive allosteric modulator of mainly α1/4/6βδ subtypes of GABA A Rs 153 , 235 – 239 . These extrasynaptic GABA A Rs are of increasing interest in the context of mood disorders as they are subject to dynamic genomic and hormonal regulation during puberty 167 , 168 , the ovarian cycle 169 , pregnancy 170 , as well as in response to stress 115 , 240 .
The cerebrospinal fluid (CSF) and plasma concentrations of THP are reduced compared to normal controls in drug-free depressed patients 154 – 157 , by social isolation stress in rats 241 , and in the olfactory bulbectomy model of depression of rats 229 . Moreover, SSRIs normalize THP deficits in patients 154 – 156 as well as in bulbectomized rats 150 , 229 , 242 , 243 . Plasma levels of THP are also elevated following partial sleep deprivation 244 , which has antidepressant effects 245 . In contrast to THP, plasma concentrations of THDOC are increased in patients and reduced by fluoxetine 157 . Unlike SSRIs or sleep deprivation, the TCA imipramine 227 , 233 , repetitive transcranial magnetic stimulation 246 and ECT 247 do not affect THP plasma concentrations, suggesting that THP is not universally involved in antidepressant mechanisms. These measurements, however, have yet to be repeated in brain to be conclusive.
In addition to drug therapies, cognitive behavioral therapy 248 and ECT 249 ameliorate cortical GABA deficits in patients. ECT is thought to further enhance GABAergic transmission through an increase in cortical expression of GABA A Rs 250 . Lastly, noradrenergic and serotonergic neurons in the locus coeruleus and raphe nucleus, respectively, are subject to GABAergic control 251 , 252 . In particular, reduced GABAergic inhibition of serotonergic neurons is a developmental risk factor for anxiety and mood disorders, as evidenced by anxiety-and depression-related behavior of mice in which the serotonin transporter was inactivated genetically (KO mice) 253 – 255 or pharmacologically 256 in early life. The collective information on the mechanisms of different antidepressant therapies and their effects on GABA release, neurosteroids synthesis and GABA A R expression and function indicate that enhancing GABAergic transmission lies at the core of both pharmacological and non-pharmacological antidepressant therapies.
- GABAergic control of neurogenesis, a target of antidepressant drug treatment
Mechanisms that regulate the production, maturation and survival of adult-born granule cell in the hippocampus (dentate gyrus) have become a focus of research on mood disorders since it was shown in rodents that these processes are enhanced by ADs 257 – 260 and required for many of the AD-induced behavioral responses 259 , 261 – 266 . Conversely, deficits in neurogenesis are a hallmark of genetic and stress-induced animal models of depression 108 , 133 , 267 – 269 and thought to underlie hippocampal atrophy observed in chronically depressed patients 24 , 26 , 27 , 105 , 139 , 270 – 277 . The production of adult-born granule cells is unaffected by serotonin depletion 278 , 279 . Moreover, noradrenaline is dispensable for normal maturation of these neurons, although it is required for normal proliferation of neural precursor cells 278 , 280 . Lastly, we are unaware of any conclusive evidence that monoamine transmitter receptors are expressed on replicating neural progenitors or on immature neurons. The collective evidence suggests that deficits in monoaminergic neurotransmitter systems are unlikely to represent principal culprits of anxiety- and depression-related deficits in hippocampal neurogenesis. By contrast, GABAergic signaling through GABA A Rs has emerged as an essential mechanism that controls proliferation, maturation and survival not only of adult-born neurons in the hippocampus 200 , 201 but also for analogous processes in the postnatal subventricular zone of rodents that replenishes interneurons of the olfactory bulb 281 , 282 and for embryonic neural progenitors that give rise to neurons of the neocortex 283 [for review see 284 , 285 ].
GABAergic mechanisms that control adult hippocampal neurogenesis
GABA A Rs have mainly hyperpolarizing effects on the membrane potential of mature neurons. By contrast, GABA-mediated activation of GABA A Rs is depolarizing and excitatory in proliferating neural progenitors and immature postmitotic neurons 281 , 283 , 285 – 288 ( Figure 2 ). The transition from GABA A R-mediated depolarization to hyperpolarization during the maturation of neurons is triggered by a developmental switch in gene expression of the two Cl − transporters NKCC1 and KCC2, which leads to a gradual shift in the membrane reversal potential of chloride to more negative values. The negative shift of the Cl − reversal potential in turn changes the direction of GABA A R-mediated currents from depolarizing (inward) in neural progenitors and immature neurons to mostly hyperpolarizing (outward) in mature neurons. Importantly, this switch is essential for normal structural and functional maturation and network integration of adult-born granule cells 201 . Short-term enhancement of GABA A R function with barbiturates accelerates the differentiation of proliferating neural progenitor cells and thereby depletes the pool of dividing cells that represents the source of adult born neurons 200 , 281 . In agreement with negative effects of GABAergic inputs on proliferation of new hippocampal neurons, co-administration of fluoxetine with the BZ diazepam negates the effect on proliferation observed with fluoxetine alone 289 . In addition to these effects on proliferating progenitors, GABA-mediated excitation of postmitotic immature neurons results in activation of low threshold T-type Ca 2+ channels 290 , higher threshold L-type Ca 2+ -channels 291 – 294 , and NMDARs 295 . The ensuing increase in intracellular Ca 2+ results in activation of diverse kinases 296 (e.g. CaMKII, PKC, PKA), all of which can phosphorylate Ser133 of the DNA-binding transcription factor CREB (cAMP response element binding protein) and promote the dendritic maturation and survival of these neurons 258 , 297 – 299 ( Figure 2 ).

A. GABA A Rs in immature neurons conduct an inward current (Cl ions moving out of the cell) due to the more positive Cl − reversal potential in these cells. The ensuing membrane depolarization facilitates Ca 2+ entry through V-gated ion channels such as the T-type and L-type voltage gated Ca 2+ channels, and in more mature neurons also NMDARs. The cytoplasmic increase in Ca 2+ results in an increased activity of protein kinases (CaMKII, PKC, PKA, others) that phosphorylate CREB on Ser133. Phosphorylated CREB translocates to the nucleus where it activates a number of target genes including that encoding BDNF. B. Increased production and release of BDNF acts on GABAergic terminals and promotes the release of GABA by TrkB/MAPK-mediated phosphorylation of synapsin and mobilization of GABA-containing vesicles, and by activation of P/Q-type voltage-gated Ca 2+ channels that activate the neurotransmitter release machinery. C, Monoamine transmitters, which are presumed to be elevated in the hippocampus upon AD treatment, act on presynaptic β-adrenergic and 5-HTRs that activate voltage-gated Ca 2+ channels on terminals and soma of GABAergic interneurons. D, Some effects of monoamine transmitters may be mediated by GPCRs on granule cells. However, the expression of these receptors on neural progenitors and immature granule cells has not been documented.
CREB mediates GABAergic control of antidepressant-induced neurogenesis
CREB has a well-established role in learning- and memory-related synaptic plasticity 300 and is involved in hippocampus-mediated AD responses 27 , 301 , 302 and the production, maturation and survival of adult-born hippocampal neurons 258 , 297 , 299 . Consistent with a role of CREB in MDD, CREB expression is down-regulated in brain of depressed (but not schizophrenic) patients studied at autopsy and increased as part of the AD response 303 . All evidence suggests that the effects of ADs on CREB activation and maturation and survival of hippocampal neurons are indirect and downstream of increased GABA signaling via GABA A Rs 299 ( Figure 1 ). Concurrent activation of CREB and increased hippocampal neurogenesis are hallmarks of all currently used antidepressants 257 , 304 , suggesting that their mechanisms of action involve enhancement of GABAergic input to immature granule cells.
Among the transcriptional target genes of CREB, the brain derived neurotrophic factor (BDNF) is of special interest 305 – 307 . BDNF is reduced in serum of depressed 308 , 309 and bipolar patients 310 , 311 and in the dentate gyrus of chronically stressed rats 312 . Conversely, BDNF is induced upon chronic treatment with diverse classes of ADs in the hippocampus of rats 313 , 314 and patients 315 , and it is effective as an antidepressant upon central administration in rodents 316 – 319 . BDNF and its receptor TrkB are essential for normal anxiety-related behavior and for AD behavioral effects in mice 264 , 320 , 321 as well as for normal neural maturation of hippocampal granule cells 322 . Importantly, BDNF is not only a target downstream of excitatory GABAergic transmission but through activation of TrkB receptors on GABAergic terminals serves to promote GABA release 323 , 324 ( Figure 2 ). Thus, BDNF enables a positive feedback loop that upregulates GABAergic signaling, which explains its essential role for normal neural maturation. A related BDNF- and GABA-mediated mechanism protects mature neurons from posttraumatic injury 325 . Currently used AD therapies 314 and ECT all enhance the expression of BDNF 313 , suggesting that these therapies might include enhancement of GABAergic transmission. However, the positive feedback relationship between GABA A R activation, BDNF expression and GABA release may be self-limited to immature neurons (and possibly other neurons with high intracellular Cl − concentrations) as BDNF also promotes the expression of KCC2, which diminishes and eventually eliminates GABAergic depolarization 326 , 327 . Indeed, in contrast to chronic effects of BDNF in immature neurons, acute effects of BDNF at synapses of mature hippocampal pyramidal cells reduce GABAergic transmission 328 – 332 by acting at postsynaptic TrkB receptors that act through PKC and PI-3 kinase-dependent signaling pathways and reduce the surface stability of GABA A Rs 329 , 332 . Moreover, unlike in immature neurons, GABAergic input to adult neurons reduces expression of BDNF 333 .
The neural maturation deficit of dentate gyrus granule cells of BDNF-depleted mice 322 is reminiscent of similar cellular deficits in GABA A R γ2 +/− mice (see below). However, unlike the depressive-like phenotype of γ2 +/− mice detailed further below, mouse lines that are depleted in BDNF or TrkB, do not reliably show behavioral signs of depression, probably reflecting opposing functions of BDNF in the ventral tegmental area (VTA) and nucleus accumbens vs. hippocampus 264 . Moreover, AD-mediated increases in BDNF do not correlate with behavioral effects induced by BDNF administered to different brain regions 334 . Whereas BDNF deficits alone cannot explain the depressive-like phenotypes of GABA A R-deficient mice, a hypomorphic human allele of BDNF (BDNF Val66Met ) is known to interact with environmental stress factors to increase the vulnerability for depression in people 335 – 337 . Preclinical experiments discussed further below suggest that these stress factors involve GABA A R deficits.
The anxiolytic effects of BZs remain intact even when hippocampal neurogenesis has been blocked 263 . This observation and the fact that BZs, unlike ADs, are effective as anxiolytics on acute treatment, indicate that the cellular substrate for anxiolytic effects of BZs is distinct from the one that mediates anxiolytic effects of ADs. Nevertheless, classical BZs are predicted to promote GABA/CREB/BDNF signaling and maturation of adult-born hippocampal neurons. However, drugs that potentiate the function of GABA A Rs do not only promote the maturation of immature neurons, they also seem to accelerate the cell cycle exit of proliferating neural progenitor cells, which delimits the pool of replicating cells and negatively affects neurogenesis 200 , 281 . These putative antagonistic effects of BZs on the total pool of immature dentate gyrus granule cells may explain the limited efficacy of BZs as antidepressants. GABA A R subtype-specific ligands that act selectively on certain GABA A R subtypes might circumvent this limitation. For example, the sedative hypnotic eszopiclone has BZ-like effects mainly on α2βγ2 and α3βγ2 subtypes of GABA A Rs 338 and promotes the survival of adult born hippocampal granule cells in rats without affecting proliferation 339 , 340 . In addition, eszopiclone has promise as a novel non-monoaminergic antidepressant in patients 187 – 189 , 341 .
GABA A R-deficient mice as animal models of depression
Gaba a r γ2 subunit deficient mice and the function of postsynaptic subtypes of gaba a rs, gabaergic deficits cause depressive-like behavioral and cognitive deficits.
The evidence for a role of GABAergic transmission summarized thus far does not prove a causal relationship between GABAergic deficits and depressive disorders. However, corresponding evidence is now available from mice engineered to model depressive disorders. In particular, mice rendered heterozygous for the γ2 subunit (γ2 +/− ) of GABA A Rs have been characterized as an animal model of anxious depression that includes anxious- and depressive-like emotional behaviors in eight different tests 49 , 108 , 342 (for a summary of phenotypes see Table 2 ). The γ2 +/− model is based on a modest functional deficit in postsynaptic GABA A Rs, as evidenced by unaltered GABA A R numbers but reduced punctate immunofluorescent staining representative of postsynaptic GABA A R subtypes and loss of GABA A R BZ binding sites ranging from 6% (amygdala) to 35% (hippocampus) of GABA A Rs, depending on brain region 342 . The magnitude of this deficit is comparable to GABA A R deficits observed in rodents that had been subjected to maternal deprivation stress 106 , 107 , suggesting it is within the pathophysiological range triggered by adverse environments that are implicated in the etiology of mood disorders. The phenotype of γ2 +/− mice includes heightened neophobia and behavioral inhibition to naturally aversive situations 342 , reduced escape attempts under highly stressful conditions 108 , as well as anhedonia-like effects 49 that mimic core symptoms of anxious melancholic depression. Lastly, γ2 +/− mice exhibit selective cognitive deficits such as an attentional bias for threat cues and impaired ambiguous cue discrimination 342 , which are reminiscent of cognitive impairments described in people at risk of or suffering from depression 343 – 348 , and principally attributed to the hippocampus 349 and frontal and cingulate cortex 350 , 351 .
Juxtaposition of phenotypes of Major Depressive Disorder and GABA A R γ2 +/− mice.
GABAergic deficits decrease the survival of adult born hippocampal neurons
Consistent with the hypotheses that depressive disorders represent chronic deficits in neurotrophic support 352 and that GABAergic signaling has trophic function 353 , the γ2 +/− model shows normal proliferation of neural precursor cells but reduced survival of adult-born hippocampal granule cells 108 . The manifestation of this neurogenesis deficit in three different global and conditional γ2-deficient mouse lines is correlated with development of anxious depressive behavior 108 , suggesting that altered neurogenesis and behavioral phenotypes are causally linked.
GABAergic deficits cause HPA axis hyperactivity and increases responsiveness to antidepressant drugs
The neuroendocrine phenotype of γ2 +/− mice includes constitutively elevated serum corticosterone and increased behavioral and endocrine sensitivity to treatment with ADs compared to wild-type mice 49 , which are known characteristics of severely depressed patients 119 , 354 . Selective heterozygous inactivation of the γ2 gene in the developing telencephalic forebrain (including hippocampus and frontal cortex, induced around embryonic day10) is sufficient to induce HPA axis hyperactivity 49 and altered behavior 108 , indicating that the causative GABAergic deficit in these mice is extra-hypothalamic ( Figure 1 ). Glucocorticoids are known to reduce expression of GABA A Rs in the forebrain, particularly in the frontal cortex and ventral hippocampus 114 , 130 , 355 . Moreover, recent evidence indicates that chronic but not acute stress results in loss of parvalbumin positive hippocampal interneurons 131 . Corresponding losses of interneurons in γ2 +/− mice might further enhance GABAergic deficits of γ2 +/− mice and amplify the observed defects in hippocampal neurogenesis. Defects in hippocampal neurogenesis in turn are sufficient to cause HPA axis hyperactivity 135 . Thus, GABA A R deficits in the telencephalon including especially the frontal cortex and hippocampus may be both a cause for, and a consequence of, HPA axis hyperactivity, a feature that may initiate a self-perpetuating feedback loop that amplifies GABAergic deficits, with HPA axis hyperactivity serving as a critical link 49 ( Figure 1 ).
GABAergic deficits cause increased therapeutic efficacy of desipramine compared to fluoxetine
The selective norepinephrine reuptake inhibitor desipramine faithfully reverses both the anxious, depressive-like and anhedonia-like behavioral phenotypes, as well as the elevated serum corticosterone concentrations of γ2 +/− mice 49 . By contrast, fluoxetine shows merely anxiolytic-like activity and fails to normalize depression-related behavior and HPA axis function of γ2 +/− mice. The qualitatively lesser response of γ2 +/− mice to fluoxetine than desipramine is reminiscent of severe subtypes of anxious depressive disorders including melancholic depression, which tend to show greater responsiveness to TCAs than fluoxetine 356 – 363 . Similar to the γ2 +/− model, clinical evidence indicates that elevated basal activity of the HPA axis is linked to poor responsiveness to fluoxetine in patients 356 , 364 , 365 , whereas normalization of HPA axis function by antidepressants is associated with remission from depression 120 , 366 .
The γ2 +/− model shows selective vulnerability to mood disorders during early life
GABAergic transmission acts as key regulator of brain development as indicated by its roles in neurogenesis 201 , neural migration 367 , maturation 108 , and circuit formation 287 , 368 , 369 . In order to delineate the developmental time course and brain regions responsible for the anxious depressive phenotype of γ2 +/− mice, the behavioral and endocrine consequences of γ2 subunit deficits were analyzed in two different conditional mutant strains (Cre-loxP system) 49 , 108 . Mice whose GABA A R deficit is initiated during embryogenesis but limited to the telencephalon were found to replicate the behavioral phenotype and HPA axis hyperactivity of global KO mice, showing that HPA axis hyperactivity can develop independently of primary GABA A R deficits in the hypothalamus 49 . By contrast, delayed inactivation of the γ2 gene during adolescence leads to developmentally delayed HPA axis hyperactivity, which is not accompanied by anxiety or depression-related behaviors 49 , 108 . These data suggest that the anxious depressive-like phenotype of γ2 +/− mice is caused by a developmental GABAergic deficit, whose sequelae include inadequate neurotrophic support in the hippocampus and chronic HPA axis activation. This scenario is consistent with heightened vulnerability to anxiety and mood disorders in people during early life 100 – 104 . In sum, the GABA A R γ2 +/− mouse model includes behavioral, cognitive, cellular, neuroendocrine and developmental dimensions as well as antidepressant drug response characteristics expected of an animal model of melancholic depression and demonstrates that GABA A R deficits can be causative for all these phenotypes.
GABA A R δ subunit-deficient mice and the function of extrasynaptic subtypes of GABA A Rs
Pregnancy and parturition are associated with marked fluctuations in neuroactive steroids, which are linked to changes in mood and anxiety level and known to act mainly through δ subunit-containing, nonsynaptic GABA A R subtypes. Failures of this neuroendocrine system to adapt to rapid changes in ovarian and adrenal hormone level are implicated in postpartum depression (PPD) and postpartum psychosis as evidenced by studies in rodents. Increased brain concentrations of neuroactive steroids during pregnancy of the rat are followed by a sudden drop to control levels within two days of delivery 370 . In rat cortex, late stage pregnancy shows decreased expression of the γ2 and α5 subunits of GABA A Rs and a corresponding reduction in GABA A R function, which rebounds after delivery 371 . In dentate gyrus granule cells and CA1 pyramidal cells, pregnancy of rats is associated with gradually increased and decreased expression of the δ and γ2 subunits of GABA A Rs, respectively, and this effect is normalized within 7 days of delivery 166 . Parturition is further associated with a rapid and transient increase in expression of the α4 subunit in the same cells 166 . The change in GABA A R subunit composition during pregnancy is associated with increased tonic GABAergic inhibition compared to neurons analyzed during estrus and dependent on de novo neurosteroid synthesis 166 .
Pregnancy in mice, unlike in rats, produces a significant downregulation of both the γ2 and δ subunits and corresponding reductions in phasic and tonic GABAergic currents recorded from hippocampal granule cell neurons 170 . Reduced expression of GABA A Rs is thought to compensate for gonadal neurosteroid-mediated increases in GABA A R activity during pregnancy. Postpartum, the expression of GABA A R subunits and the phasic and tonic GABAergic currents recorded from granule cells rebound rapidly to levels found in virgin females. Interestingly, GABA A R δ subunit KO mice, which are unable to adjust expression of δ-containing GABA A Rs show drastic deficits in GABAergic tonic inhibition specifically postpartum, that is associated with anxiety and depression-related behavior as well as abnormal maternal behavior. The pathology of δ subunit KO mice thereby mirrors the symptoms of psychotic PPD 170 .
Dynamic changes in neurosteroid synthesis and GABA A R subunit expression also occur during the estrus cycle, and alterations in these mechanisms are implicated in the etiology of premenstrual dysphoric disorder (PMDD) 169 , 372 . Elevated expression of α4βδ receptors in late diestrus (high-progesterone phase) of the mouse causes increased tonic inhibition of dentate gyrus granule cells along with reduced anxiety 169 . Reduced expression of the δ subunit during estrus is paralleled by upregulation of γ2-containing GABA A Rs, which are comparatively insensitive to neurosteroids. Pharmacological blockade of neurosteroid synthesis from progesterone inhibits cyclic changes in GABA A R subunit expression and neural plasticity while the progesterone receptor antagonist RU486 has no effect, indicating that neurosteroid synthesis rather than nuclear progesterone receptor activation underlies hormone-mediated neural plasticity 115 . Consistent with this interpretation, upregulation of α4βδ receptors and tonic inhibition in hippocampal granule cells can be induced by treatment with THDOC or by acute stress, a condition known to increase neurosteroid levels 115 . Estrus cycle-associated changes in the expression of α4βδ receptors have also been shown in the periaqueductal gray matter of female rats 165 , indicating that neurosteroid–induced plasticity is not limited to the dentate gyrus. In addition to the role of neurosteroids in regulating GABA A R subunit gene expression and as allosteric modulators of α4βδ receptors, neurosteroids have been shown to regulate protein kinase C (PKC)-mediated phosphorylation of GABA A Rs 373 . PKC is known to regulate the cell surface accumulation of GABA A Rs and GABAergic inhibition 374 . In sum, anomalous regulation of α4βδ receptors by neurosteroids at the level of gene expression, channel gating and/or receptor trafficking is implicated in the etiology of PPD and PMDD.
- Conclusions, limitations, and outlook
The collective evidence summarized here indicates that reduced concentrations of GABA and altered expression of GABA A Rs are common abnormalities observed in MDDs. GABAergic transmission is vital for the control of stress and impaired by chronic stress, the most important vulnerability factor of MDD. Currently used antidepressants, which are designed to augment monoaminergic transmission, have in common that they ultimately serve to enhance GABAergic transmission. GABAergic excitation of immature neurons in the dentate gyrus has been identified as a key mechanism that provides trophic support and controls the dendritic maturation and survival of neurons, a process that serves as a molecular and cellular substrate of antidepressant drug action. Lastly, comparatively modest deficits in GABAergic transmission are sufficient to cause most of the cellular, behavioral, cognitive and pharmacological sequelae expected of an animal model of major depression. GABAergic transmission is further subject to dynamic regulation by estrus- and pregnancy-associated changes in steroid hormone synthesis and altered expression of extrasynaptic GABA A Rs that may contribute preferentially to female-specific risk factors of mood disorders and explain the increased prevalence of MDD in the female population. The behavioral phenotypes in GABA A R γ2 +/− and δ subunit knockout mice suggest that deficits in both synaptic and nonsynaptic GABAergic transmission can contribute to depressive disorders.
Despite remarkable recent progress we are left with a number of significant gaps in understanding. GABAergic deficits are not unique to MDD but similarly implicated in a number of other neuropsychiatric disorders, especially schizophrenia 375 , 376 . The question arises whether and how GABAergic deficits can help to differentiate between these different disorders. Moreover, the mechanisms that lead to initial GABAergic deficits remain poorly understood and they are so far not explained by mutations or functional polymorphisms in genes intimately involved in GABAergic transmission. We have listed a number of reasons that explain why currently available GABA potentiating drugs are ineffective as antidepressants, yet it remains to be established whether next generation GABAergic drugs that are more selective for GABA A Rs expressed in corticolimbic circuits affected in depression exhibit more convincing efficacy as antidepressants. Furthermore, a number of aspects of major depressive disorders are not know to involve GABAergic deficits. For example, there is increasing preclinical evidence that resilience to stress and stress-induced neuropsychiatric disorders including depression are subject to epigenetic mechanisms 377 , yet there is little evidence for epigenetic regulation of GABAergic transmission. Transcriptional and immunohistochemical alterations in brain of depressed patients suggest links between depressive disorders and inflammation, apoptosis 378 and oligodendrocyte dysfunction 379 , 380 , but none of these have been linked to GABAergic deficits. Future research should address these gaps in understanding and lead the path to improved antidepressant therapies that strive to correct the causal neurochemical imbalances rather than merely the symptoms of depression.
- Acknowledgments
We thank Byron Jones, Pam Mitchell and Casey Kilpatrick for critical reading of the manuscript. Research in the Luscher laboratory is supported by grants MH62391, MH60989 and RC1MH089111 from the National Institutes of Mental Health (NIMH), and a grant from the Pennsylvania Department of Health using Tobacco Settlement Funds. The contents of this review are solely the responsibility of the authors and do not necessarily represent the views of the NIMH or the NIH. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.
Conflicts of Interest. The authors declare no conflict of interest
- Structure of GABAARs
- Modulation of GABAARs by stress: a major risk factor of depressive disorders
- GABAAR-deficient mice as animal models of depression
Full text links
Read article at publisher's site: https://doi.org/10.1038/mp.2010.120
Citations & impact
Impact metrics, citations of article over time, alternative metrics.

Smart citations by scite.ai Smart citations by scite.ai include citation statements extracted from the full text of the citing article. The number of the statements may be higher than the number of citations provided by EuropePMC if one paper cites another multiple times or lower if scite has not yet processed some of the citing articles. Explore citation contexts and check if this article has been supported or disputed. https://scite.ai/reports/10.1038/mp.2010.120
Article citations, the α-7 nicotinic receptor positive allosteric modulator alleviates lipopolysaccharide induced depressive-like behavior by regulating microglial function, trophic factor, and chloride transporters in mice..
Alzarea S , Khan A , Ronan PJ , Lutfy K , Rahman S
Brain Sci , 14(3):290, 19 Mar 2024
Cited by: 0 articles | PMID: 38539677 | PMCID: PMC10968594
The Ribosome Hypothesis: Decoding Mood Disorder Complexity.
Sharma V , Swaminathan K , Shukla R
Int J Mol Sci , 25(5):2815, 29 Feb 2024
Cited by: 0 articles | PMID: 38474062 | PMCID: PMC10931790
Sleep disorders cause Parkinson's disease or the reverse is true: Good GABA good night.
Al-Kuraishy HM , Al-Gareeb AI , Albuhadily AK , Elewa YHA , Al-Farga A , Aqlan F , Zahran MH , Batiha GE
CNS Neurosci Ther , 30(3):e14521, 01 Mar 2024
Cited by: 0 articles | PMID: 38491789 | PMCID: PMC10943276
Prefrontal Cortex Cytosolic Proteome and Machine Learning-Based Predictors of Resilience toward Chronic Social Isolation in Rats.
Filipović D , Novak B , Xiao J , Tadić P , Turck CW
Int J Mol Sci , 25(5):3026, 06 Mar 2024
Cited by: 0 articles | PMID: 38474271 | PMCID: PMC10931671
A Bright Future? A Perspective on Class C GPCR Based Genetically Encoded Biosensors.
Otanuly M , Kubitschke M , Masseck OA
ACS Chem Neurosci , 15(5):889-897, 21 Feb 2024
Cited by: 0 articles | PMID: 38380648
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
GABAergic control of depression-related brain states.
Luscher B , Fuchs T
Adv Pharmacol , 73:97-144, 14 Jan 2015
Cited by: 64 articles | PMID: 25637439 | PMCID: PMC4784429
Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state.
Fuchs T , Jefferson SJ , Hooper A , Yee PH , Maguire J , Luscher B
Mol Psychiatry , 22(6):920-930, 08 Nov 2016
Cited by: 90 articles | PMID: 27821870 | PMCID: PMC5422144
Altered γ-aminobutyric acid neurotransmission in major depressive disorder: a critical review of the supporting evidence and the influence of serotonergic antidepressants.
Pehrson AL , Sanchez C
Drug Des Devel Ther , 9:603-624, 19 Jan 2015
Cited by: 66 articles | PMID: 25653499 | PMCID: PMC4307650
Bidirectional Homeostatic Regulation of a Depression-Related Brain State by Gamma-Aminobutyric Acidergic Deficits and Ketamine Treatment.
Ren Z , Pribiag H , Jefferson SJ , Shorey M , Fuchs T , Stellwagen D , Luscher B
Biol Psychiatry , 80(6):457-468, 13 Feb 2016
Cited by: 58 articles | PMID: 27062563 | PMCID: PMC4983262
GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action.
Sanacora G , Saricicek A
CNS Neurol Disord Drug Targets , 6(2):127-140, 01 Apr 2007
Cited by: 80 articles | PMID: 17430150
Funding
Funders who supported this work.
NIMH NIH HHS (8)
Grant ID: MH62391
14 publication s
Grant ID: R01 MH099851
27 publication s
Grant ID: RC1MH089111
2 publication s
Grant ID: MH60989
11 publication s
Grant ID: RC1 MH089111
Grant ID: R01 MH062391
19 publication s
Grant ID: R56 MH062391
17 publication s
Grant ID: R01 MH060989
10 publication s
Europe PMC is part of the ELIXIR infrastructure
The GABA system in anxiety and depression and its therapeutic potential
Affiliation.
- 1 Institute of Pharmacology, University of Zurich and Department of Chemistry and Applied Biosciences, ETH Zurich, Winterthurerstr. 190, CH-8057 Zurich, Switzerland. [email protected]
- PMID: 21889518
- DOI: 10.1016/j.neuropharm.2011.08.040
In the regulation of behavior, the role of GABA neurons has been extensively studied in the circuit of fear, where GABA interneurons play key parts in the acquisition, storage and extinction of fear. Therapeutically, modulators of α(2)/α(3) GABA(A) receptors, such as TPA023, have shown clinical proof of concept as novel anxiolytics, which are superior to classical benzodiazepines by their lack of sedation and much reduced or absent dependence liability. In view of the finding that anxiety disorders and major depression share a GABAergic deficit as a common pathophysiology, the GABA hypothesis of depression has found increasing support. It holds that α(2)/α(3) GABA(A) receptor modulators may serve as novel antidepressants. Initial clinical evidence for this view comes from the significantly enhanced antidepressant therapeutic response when eszopicole, an anxiolytic/hypnotic acting preferentially on α(2)/α(3) and α(1) GABA(A) receptors, was coadministered with an antidepressant. This effect persisted even when sleep items were not considered. These initial results warrant efforts to profile selective α(2)/α(3) GABA(A) receptor modulators, such as TPA023, as novel antidepressants. In addition, GABA(B) receptor antagonists may serve as potential antidepressants. This article is part of a Special Issue entitled 'Anxiety and Depression'.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Publication types
- Antidepressive Agents / therapeutic use*
- Anxiety / drug therapy*
- Anxiety / metabolism*
- Anxiety / pathology
- Depression / drug therapy
- Depression / metabolism*
- Depression / pathology
- GABA Agonists / therapeutic use
- Neural Pathways / drug effects
- Neural Pathways / physiology
- Pyridazines / therapeutic use
- Receptors, GABA / metabolism
- Triazoles / therapeutic use
- gamma-Aminobutyric Acid / metabolism*
- 7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo(4,3-b)pyridazine
- Antidepressive Agents
- GABA Agonists
- Pyridazines
- Receptors, GABA
- gamma-Aminobutyric Acid
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 29, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Polyamorous youth report facing stigma, heightened levels of depression
by Sara Zaske, Washington State University

While increasingly visible among adults, polyamory also exists among adolescents, and as a new study indicates, so does the stigma that can come with it.
A Washington State University study of 323 youth ages 12 to 17 at an LGBTQ+ summer camp found that 54, or about 16.7%, identified as polyamorous or ambiamorous, meaning they were open to either monogamous or polyamorous relationships. These "poly" and "ambi" youth reported higher levels of depressive symptoms than their LGBTQ+ peers.
The study, one of the first to investigate polyamorous relationships in youth, was published in the journal Psychology & Sexuality .
"It was notable that many of the polyamorous teens said they wouldn't feel safe being out in their home communities," said study author Traci Gillig, a WSU researcher. "They felt like they would be misunderstood or that people have stereotypes or judgments around what it means for them to be poly, like that they are promiscuous or don't perceive cheating as a problem."
Polyamory is a relationship structure that involves having more than one romantic partner at the same time with the consent and knowledge of all the partners, so as with monogamous relationships, the secrecy of cheating is considered a breach of trust. Again similar to monogamy, polyamory is primarily about relationships and does not necessarily have to involve sex at all.
This study was limited to a camp for LGBTQ+ youth called Brave Trails, which likely indicates the adolescents came from more accepting families, Gillig noted. However, 30 adolescents still reported they either would not feel safe, or felt unsure if they would be safe, if they were open about being poly in their home communities.
Gillig said it was encouraging that many also felt they would be supported, and 16 of the 54 poly or ambi campers said they were open about it at home.
Adult polyamory has been gaining attention in the news media and on TV with shows that feature poly people on Netflix and Showtime. It has also been the subject of research, which has found that more than 20% of adults have engaged in consensually non-monogamous relationships like polyamory. Another study also found that some poly adults began to understand their identity as poly when they were adolescents.
For this study, participants filled out questionnaires before and at the end of the camp, which included assessments of anxiety and depressive symptoms. They also answered questions about their preferred relationship structure and how comfortable they felt being open with others about it.
The survey allowed campers to write in explanations, and some who felt less safe said that being poly was "a touchy subject" and that even those who accept their LGBTQ+ identity would not be okay with it.
The poly and ambi kids as well as all the adolescents in the study showed improved mental health after experiencing the accepting environment of the LGBTQ+ camp, and Gillig emphasized that support is key for young people who have a marginalized identity.
"Youths' experience with being polyamorous or ambiamorous is similar to being LGBTQ+ in that if they perceive that they won't be supported, then they're not as likely to disclose their identity at home. We know from research with queer youth that this can cause elevated levels of depressive symptoms," she said. "My hope is that parents would have an open mind, if their child comes to them and expresses that they identify as polyamorous or if they have questions about it."
Explore further
Feedback to editors
Microarray patches safe and effective for vaccinating children, trial shows
6 hours ago

Healthy lifestyle may offset effects of life-shortening genes by more than 60%

Fentanyl inhalation may cause potentially irreversible brain damage, warn doctors

Frequent teen vaping might boost risk of toxic lead and uranium exposure
Study in Haiti suggests early-onset heart failure is prevalent form of heart disease in low-income countries
8 hours ago

AI algorithms can determine how well newborns nurse, study shows
9 hours ago

Kaposi sarcoma discovery and mouse model could facilitate drug development
Immune cell interaction study unlocks novel treatment targets for chikungunya virus

New method rapidly reveals how protein modifications power T cells
Protein responsible for genetic inflammatory disease identified
10 hours ago
Related Stories
Frequent social media use influences depressive symptoms over time among lgbtq youth.
Jul 23, 2020

What's a polycule? An expert on polyamory explains
Dec 1, 2022

Polyamorous relationships can have as many benefits as monogamous ones, shows research
Apr 3, 2023

How non-monogamous relationships find success
Jun 28, 2017

Talking to teens about sex: Advice for parents on when, how, what to say and why it's so important
16 hours ago

Polyamorous relationships under severe strain during the pandemic
Feb 11, 2021
Recommended for you

Nature's nudge: Study shows green views lead to healthier food choices
12 hours ago

Researchers look at genetic clues to depression in more than 14,000 people
13 hours ago

Brain function of older adults catching up with younger generations, finds study
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
Increasing evidence points to an association between major depressive disorders (MDDs) and diverse types of GABAergic deficits. In this review, we summarize clinical and preclinical evidence supporting a central and causal role of GABAergic deficits in the etiology of depressive disorders. Studies of depressed patients indicate that MDDs are ...
Introduction. Major depressive disorder (MDD) represents a complex neuropsychiatric syndrome with a lifetime prevalence of approximately 17% of the population worldwide 1.It exhibits high comorbidity with anxiety disorders, with 50-60% of depressed patients reporting a lifetime history of anxiety disorders, and many anxiety disorder patients showing a history of treatment for depression 2-9.
GABA deficits in depression. The strongest evidence that GABAergic deficits may contribute to depressive disorders is based on reduced GABA levels in plasma 53, 54 and cerebrospinal fluid 55 or in ...
The GABAergic deficit hypothesis of major depressive disorder B Luscher1,2,3,4, Q Shen2,4 and N Sahir1,4 1Departments of Biology, ... depression.2-9 Antidepressant drug (AD) treatments
The GABAergic deficit hypothesis proposes that defects in GABAergic neural inhibition causally contribute to the common phenotypes of MDD, and, conversely, the efficacy of an ADT may be linked to ...
The GABAergic deficit hypothesis of major depressive disorder. Clinical and preclinical evidence supporting a central and causal role of GABAergic deficits in the etiology of depressive disorders is summarized and the GABAergic hypothesis of MDD suggests that alterations in GABAergic transmission represent fundamentally important aspects of the ...
GABAergic Transmission in Relation to the Neurotrophic Deficit Hypothesis of MDD. The neurotrophic deficit hypothesis of MDD is principally based on brain imaging studies that have revealed brain volume reductions in limbic regions implicated in depression, including primary areas of pathology such as the hippocampus and prefrontal cortex.
For decades, the monoaminergic deficit hypothesis of depression provided the conceptual framework to understand the pathophysiology of MDD. However, accumulating evidence suggests that MDD and chronic stress are associated with an imbalance of excitation-inhibition (E:I) within the PFC, generated by a deficit of inhibitory synaptic transmission ...
GABAergic Transmission in Relation to the Neurotrophic Deficit Hypothesis of MDD. The neurotrophic deficit hypothesis of MDD is principally based on brain imaging studies that have revealed brain volume reductions in limbic regions implicated in depression, including primary areas of pathology such as the hippocampus and prefrontal cortex.
In view of the finding that anxiety disorders and major depression share a GABAergic deficit as a common pathophysiology, the GABA hypothesis of depression has found increasing support. ... In depression, the GABA hypothesis is based on four elements 1) Patients show a cortical GABA deficit which is reversed by chronic SSRI treatment. 2) ...
Major depressive disorder (MDD) is a debilitating illness characterized by neuroanatomical and functional alterations in limbic structures, notably the prefrontal cortex (PFC), that can be precipitated by exposure to chronic stress. For decades, the monoaminergic deficit hypothesis of depression provided the conceptual framework to understand the pathophysiology of MDD. However, accumulating ...
The hypothesis of GABA dysfunction has long been considered as the important pathological mechanism of depression. 4, 5 The evidence from clinical trials indicated that GABAergic neurotransmission and GABA content were substantially decreased in depressed patients. 6-8 Additionally, GABAergic interneuron is a leading cause of alteration in ...
The GABAergic deficit hypothesis of major depressive disorders (MDDs) posits that reduced γ-aminobutyric acid (GABA) concentration in brain, impaired function of GABAergic interneurons, altered expression and function of GABA A receptors, and changes in GABAergic transmission dictated by altered chloride homeostasis can contribute to the etiology of MDD.
The GABAergic deficit hypothesis of MDD presented here suggests that local GABAergic deficits in hippocampus and frontal cortex due to reduced ... Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996; 29:2-11 ...
Brexanolone, a neurosteroid antidepressant, vindicates the GABAergic deficit hypothesis of depression and may foster resilience. F1000Res. 2019; 8: 751. Crossref; Scopus (44) Google Scholar]. Chronic stress is a major risk factor for psychiatric illnesses and has been widely used to model features of mood disorders in animals . Neurosteroids ...
In view of the finding that anxiety disorders and major depression share a GABAergic deficit as a common pathophysiology, the GABA hypothesis of depression has found increasing support. It holds that α (2)/α (3) GABA (A) receptor modulators may serve as novel antidepressants. Initial clinical evidence for this view comes from the ...
A Washington State University study of 323 youth ages 12 to 17 at an LGBTQ+ summer camp found that 54, or about 16.7%, identified as polyamorous or ambiamorous, meaning they were open to either ...
GABAergic mechanisms have been generally ignored in the study of mood disorders and antldepressant drug (AD) action. Recently data have accumulated indicating that GABAergic mechanisms may be involved in both of these. 2. Mood disorders: GABA levels are reported to be low in the CSF and plasma of depressed patients and are related to mood changes.